-
PDF
- Split View
-
Views
-
Cite
Cite
William A. Petri, Rashidul Haque, Dinesh Mondal, Anwarul Karim, Imarot Hossain Molla, Abdur Rahim, Abu S. G. Faruque, Nooruddin Ahmad, Beth D. Kirkpatrick, Eric Houpt, Cynthia Snider, Prospective Case-Control Study of the Association between Common Enteric Protozoal Parasites and Diarrhea in Bangladesh, Clinical Infectious Diseases, Volume 48, Issue 9, 1 May 2009, Pages 1191–1197, https://doi.org/10.1086/597580
Close - Share Icon Share
Abstract
Background. The parasitic causes of diarrhea have historically been identified by use of microscopy; however, the use of this technique does not allow one to distinguish between subspecies or genotypes of parasites. Our objective was to determine, by use of modern diagnostic methods, the proportion of diarrhea cases in Bangladesh attributable to Cryptosporidium hominis, Cryptosporidium parvum, Entamoeba histolytica, and Giardia lamblia assemblages A and B.
Methods. A prospective case-control study was performed involving 3646 case patients (both children and adults) who presented with diarrhea to the Dhaka hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh, and 2575 control subjects with asymptomatic infection. Parasitic infection was detected by use of a stool parasite antigen test, and the parasite load and the species and/or genotypes were determined by use of polymerase chain reaction (PCR).
Results.Cryptosporidium species and E. histolytica were more prevalent in patients with acute diarrhea than in healthy control subjects, for all ages (2.1% vs. 1.4%; P=.039) and, specifically, for those 0–12 months of age (2.2% vs. 0.4%; P=.009). G. lamblia assemblage A was also more prevalent in case patients with diarrhea than in healthy control subjects (20% vs. 5%; P<.001). For case patients with diarrhea, the parasite load in feces, as measured by quantitative real-time PCR cycle threshold, was not higher that that for control subjects with asymptomatic infection. Case patients with diarrhea and cryptosporidiosis were less likely to have abdominal pain, compared with control subjects (15% vs. 37%; P<.001); case patients with amebiasis more likely to have visible blood in stool, compared with control subjects (8% vs. 1.6%; P<.001); and case patients with giardiasis more likely to be dehydrated, compared with control subjects (81% vs. 71%; P=.001).
Conclusion.E. histolytica, C. hominis, C. parvum, and G. lamblia assemblage A infections are important causes of diarrheal illness in Bangladesh.
Diarrheal diseases are a major public health problem that particulary affects children in developing countries, including Bangladesh [1]. A number of bacterial, viral, and parasitic agents have been identified in patients with acute diarrhea [2–5]. Entamoeba histolytica, Giardia lamblia, and Cryptosporidium species are the common enteric protozoal parasites generally believed to be associated with diarrhea [6, 7]. Nevertheless, the role of G. lamblia in acute diarrheal illness is still in dispute [8, 9]. Microscopy was used in the past to detect these protozoal parasites, but microscopic examination is unable to distinguish between invasive parasites, such as E. histolytica, and commensal parasites, such as Entamoeba dispar and Entamoeba moshkovskii. The use of microscopy is also less sensitive for detection of G. lamblia and Cryptosporidium species and cannot be used to distinguish between genotypes of giardia or species of cryptosporidia.
The Dhaka hospital of the International Centre for Diarrheal Disease Research, Bangladesh, treats >100,000 patients with diarrhea annually. Prior studies from the Dhaka hospital, where there is a systematic surveillance of 2% of all patients, used microscopy as the diagnostic method [10, 11]. In our study, we compared the number of isolates of E. histolytica, Cryptosporidium species, and G. lamblia recovered from patients with acute diarrheal illness with those recovered from control subjects without diarrhea, by use of antigen detection kits and PCR-based genotyping. An interim analysis of the data on G. lamblia infection from this study has been published [12].
Materials and Methods
Study population. Case patients were patients of all ages with acute diarrhea who were seeking treatment during the period from May 2004 through April 2006 at the Dhaka hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh. These patients were part of a routine systemic surveillance of 2% of all patients. Acute diarrhea was defined as ⩾3 abnormal stools within the previous 24 h, and dysentery was defined by the presence of RBCs, macrophages, or pus cells. All case patients received treatment according to the treatment guidelines at the Dhaka hospital of the International Centre for Diarrheal Disease Research, Bangladesh. Control subjects were individuals of all ages with asymptomatic infection who reported no diarrheal illness in the previous 3 months and who were enrolled in the study through the outpatient clinics of the Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Laboratory methods. Stool samples were examined by use of microscopy and cultured in Robinson's medium within 6 h after collection. Detection of the protozoa was performed by use of commercially available antigen detection kits (TechLab) for G. lamblia, Cryptosporidium species, and E. histolytica. Stool samples were plated on MacConkey agar for the detection of these protozoa, on Salmonella Shigella agar for the detection of Salmonella and Shigella, and on taurocholate-tellerite-gelatin agar for the detection of Vibrio cholerae. Stool specimens were examined for rotavirus by using an ELISA, as described elsewhere [13]. Stool specimens from healthy control subjects were not analyzed for bacterial or viral enteropathogens.
DNA was extracted only from stool specimens that tested positive for antigens. DNA was purified from 200 mg of stool by use of the QIAamp DNA Stool Mini Kit (Qiagen). G. lamblia genotypes were determined for stool specimens with positive microscopy findings or with positive antigen test results by use of a Scorpion probe–based real-time PCR assay, as described elsewhere [14]. This assay amplifies a 95- or 102-bp region of the 18S rRNA gene using species- and/or genotype-specific primers and the probe. Cryptosporidium species were determined for stool specimens with positive microscopy findings or with positive antigen test results by use of a sybergreen-based real-time PCR technique [15] that amplifies a Cryptosporidium coding sequence of unknown function (AF190627). All amplifications were performed according to these references on a real-time PCR system (iCycler; BioRad).
Informed consent was obtained from the study subjects, and if they were children, consent was obtained from their parents or guardians. The human experimentation guidelines of the US Department of Health and Human Services, the University of Virginia, and the International Center for Diarrhoeal Disease Research, Bangladesh, were followed during the course of our research.
Results
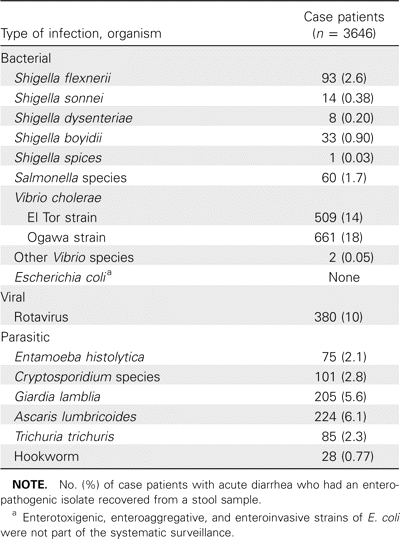
A total of 3646 case patients with acute diarrheal illness were enrolled in our study as part of the systematic surveillance of 2% of all patients admitted to the Dhaka hospital of the International Centre for Diarrheal Disease Research, Bangladesh. Of these 3646 case patients, 2086 (57%) were male, and 1560 (43%) were female. There were 1094 case patients (30%) who were 0–12 months of age, 656 (18%) who were 1–5 years of age, 292 (8%) who were 6–14 years of age, 1240 (34%) who were 15–40 years of age, and 401 (11%) who were >40 years of age. A total of 2575 healthy control subjects consented to participate in our study. Of these 2575 control subjects, 1401 (54%) were male, and 1174 (46%) were female. There were 489 control subjects (19%) who were 0–12 months of age, 670 (26%) who were 1–5 years of age, 464 (18%) who were 6–14 years of age, 747 (29%) who were 15–40 years of age, and 232 (9%) who were >40 years of age. V. cholerae was the most common pathogen isolated from diarrheal stool specimens (table 1).
Data on the enteropathogens recovered from case patients and identified from the systematic surveillance system of the International Centre for Diarrheal Disease Research, Bangladesh, at Dhaka hospital, May 2004–April 2006.
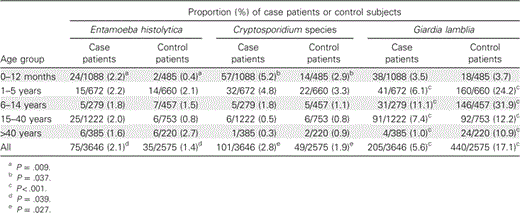
The isolation rates of Cryptosporidium species and E. histolytica among patients with acute diarrhea were 2.8% (101 of 3646 patients) and 2.1% (75 of 3646 patients), respectively. The prevalence of these parasites was significantly higher among case patients than among healthy control subjects in the first 12 months of life (table 2). Cryptosporidium parvum and Cryptosporidium hominis were present in both diarrheal and nondiarrheal stool samples at roughly equal percentages (C. hominis was detected as the sole cause of infection in 61% of case patients and in 54% of control subjects infected with cryptosporidia; mixed infections with both C. hominis and C. parvum were observed in 21% of case patients and in 17% of control subjects [P>.05; data not shown]). We have previously reported from this cohort the genotypes of 283 individuals with giardiasis; the 343 individuals shown in table 3 include these previously reported individuals [12]. G. lamblia, in general, was significantly more common among control subjects than among case patients (table 2); however, G. lamblia genotype “A” was associated with acute diarrhea (table 3).
Distribution of isolates of parasitic protozoa recovered from stool samples of case patients and control subjects, by age group.
Distribution of genotypes of Giarida lamblia isolates recovered from stool samples of case patients and control subjects, by use of antigen detection test.
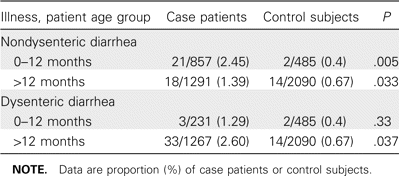
Case patients with or without dysenteric diarrhea and healthy control subjects were tested for E. histolytica (table 4). The association of E. histolytica with nondysenteric diarrhea was statistically significant for all case patients and control subjects (table 4), whereas the association of E. histolytica with dysenteric diarrhea was significantly higher for case patients >12 months of age than it was for control subjects >12 months of age. It was interesting to note that each of the stool specimens positive for E. histolytica (by use of the antigen detection test) that were obtained from 24 children 0–12 months of age were negative for all other enteric pathogens, except enterotoxigenic E. coli in 3 samples (data not shown). All of these 24 children with E. histolytica–associated diarrhea presented with loose or watery stool, had no history of bloody stools, and had no RBCs in their stool specimens.
Association of Entamoeba histolytica with diarrhea and dysentery, based on age group.
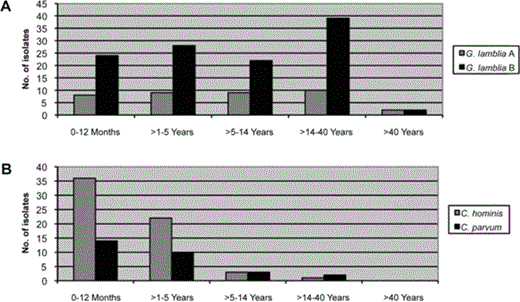
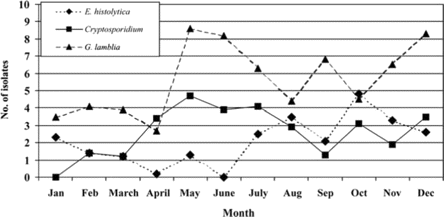
In table 2, it can been seen that the increasing age of both case patients and control subjects was associated with a decrease in the number of Cryptosporidium isolates recovered from stool samples, whereas the number of E. histolytica isolates recovered remained constant. However, the number of G. lamblia isolates recovered from stool samples peaked in the 6–14-year-old age group for both case patients and contol subjects (table 2). There was no statistically significant difference in the number of G. lamblia assemblage A isolates recovered, compared with assemblage B isolates, or in the number of C. hominis isolates recovered, compared with C. parvum isolates, between the different age groups (figure 1). E. histolytica–associated diarrhea and Cryptosporidium-associated diarrhea were equally prevalent in both sexes (data not shown). More isolates of the 3 protozoa (i.e., E. histolytica, Cryptosporidium species, and G. lamblia) were recovered from stool samples during the summer months than at other times of the year (figure 2).
The number of Giardia lamblia assemblage A isolates recovered, compared with G. lamblia assemblage B isolates, and the number of Cryptosporidium hominis isolates recovered, compared with C. parvum isolates, stratified by the different age groups of case patients and control subjects.
Seasonality of enteric protozoa in case patients with acute diarrhea, as shown by the number of isolates of Entamoeba histolytica, Cryptosporidium species, and Giardia lamblia recovered from stool samples, by month of the year.
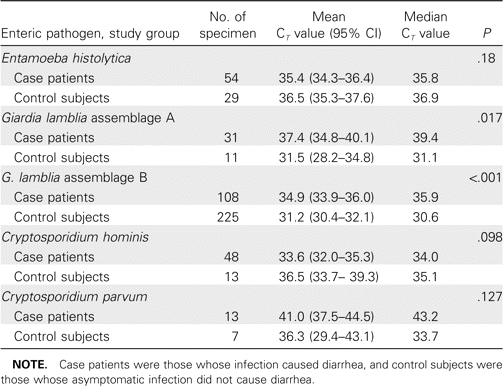
The parasite load was estimated by measuring the cycle threshold (CT) value of the individual real-time PCR assays (table 5). There was no difference between case patients and control subjects in the amount of C. parvum, C. hominis, or E. histolytica DNA per 200 mg of stool. Surprisingly, there was significantly less G. lamblia in the diarrheal stool of control subjects, as assessed by the quantitative PCR CT value (table 5) and the semiquantitative stool antigen detection test (data not shown).
Parasite load in case patients and control subjects, as measured by quantitative real-time PCR cycle threshold (CT) values, at the Dhaka hospital of the International Centre for Diarrheal Disease Research, Bangladesh, May 2004–April 2006.
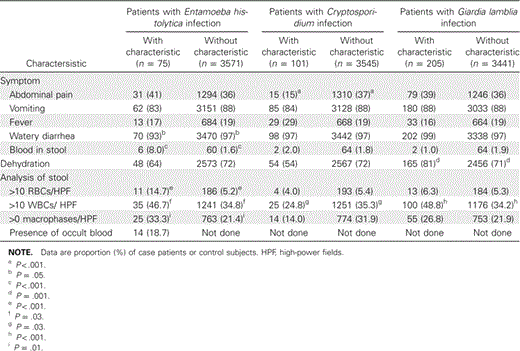
Case patients with cryptosporidiosis were less likely than other case patients to have abdominal pain, case patients with amebiasis were more likely than other case patients to have visible blood in their stool, and case patients with giardiasis were more likely to be dehydrated (table 6). Microscopic examination of the diarrheal stool specimens revealed a significantly higher amount of RBCs and macrophages in stool samples that were positive for E. histolytica (table 6).
Clinical characteristics of case patients with enteric protozoal–associated diarrhea, at the Dhaka hospital of the International Centre for Diarrheal Disease Research, Bangladesh, May 2004–April 2006.
Discussion
The application of modern molecular methods to this large prospective case-control study of parasitic diarrhea provided several insights. First, we found that enteric parasites were prevalent and an important cause of diarrhea: Cryptosporidium species, E. histolytica, and G. lamblia were identified in 10.5% of case patients with diarrhea severe enough to warrant hospital admission (table 1), and cryptosporidiosis and amebiasis were diagnosed in 7.4% of case patients with diarrhea who were in the critical first year of life, when mortality due to diarrheal disease is highest (table 2) [16]. Second, it appeared that the G. lamblia parasite load, as measured in the stool, was inversely related to diarrhea. Third, in the case of giardiasis, we extended our previous observation that assemblage A, but not assemblage B, was associated with diarrhea. Finally, we described a previously underappreciated manifestation of amebiasis, which is defined as nondysenteric diarrhea during the first year of life.
Several studies have examined E. histolytica isolates recovered from stool samples of patients with acute diarrhea but, because of the lack of a case-control study design, were unable to investigate the association of E. histolytica with nondysenteric diarrhea [7, 9]. Whenever case-control studies have been conducted, microscopy has been used for diagnosis; unfortunately, the use of microscopy does not allow one to differentiate E. histolytica from the morphologically similar and prevalent nonpathogenic parasites E. dispar and E. moshkovskii [17–22]. In our present study, we identified E. histolytica by use of a specific antigen detection test. Although E. histolytica is generally known to cause bloody diarrhea, our study demonstrated that E. histolytica could also be a cause of watery diarrhea, particularly in infants. That nondysenteric diarrhea is a common presentation of amebiasis is in agreement with the study by Samie et al. [17] involving children <2 years of age and with our own work with children 2–12 years of age [22].
Our study also confirmed the association between Cryptosporidium species and acute diarrheal illness [10, 23, 24]. Isolates of Cryptosporidium species were recovered from 1.4% of patients in a previous study performed in the same hospital in 1994 [10]. Because we used a more sensitive antigen detection test for diagnosis of cryptosporidiosis in our study, we were able to recover Cryptosporidium species from a much higher percentage of patients. In addition, we have demonstrated that both C. parvum and C. hominis were present in stool samples obtained from case patients at this hospital. However, neither of these parasites was more likely than the other to be associated with diarrhea, confirming the observations of other investigators [25–27]. The prevalence of Cryptosporidium isolates decreased with increasing patient age, which implies that long-lasting natural immunity may persist after Cryptosporidium infection in the first few years of life.
The lack of correlation—or, in the case of G. lamblia, the inverse correlation—between parasite load and symptoms of diarrhea was surprising. The emerging literature suggests that diarrhea due to infection with cryptosporidia, giardia, and entamoeba is accompanied by robust and likely deleterious production of proinflammatory cytokines, such as TNF-α [28–31]. The lack of a positive correlation between parasite load and symptoms of diarrhea is suggestive of the primary role played by the immune system in diarrheal illness that results from these infections.
The limitations of our study were that only cases of diarrhea severe enough to merit hospitalization were included and that no longitudinal follow-up was conducted, so it is likely that our study did not reflect the full spectrum of illness attributable to enteric parasites or their impact on children. For example, it might be that G. lamblia assemblage B does, in fact, cause diarrhea, only in a milder or self-limited form, or it might be that the enteric parasites have an even larger effect on children than our study indicates, in the form of more-prolonged or subclinical diarrhea. Another limitation was that case patients and control subjects were not perfectly matched: control subjects tended to be older than case patients, a fact that we corrected for by analyzing infections as a percentage of all case patients or control subjects in a given age group. However, there could be other errors introduced by the less-than-perfect matching of case patients to control subjects. Finally, inaccuracies in the diagnostic tests used or PCR inhibitors unique to diarrheal or nondiarrheal stools might have affected the measurement of parasites. We attempted to control for these factors by using both antigen detection and PCR tests on the stool samples positive for parasites.
In conclusion, the use of antigen detection and PCR-based diagnostic tests validated the importance of enteric parasites as a cause of diarrhea during the first year of life and illustrated aspects of these infections that had not been fully appreciated. This knowledge will aid in the planning of longitudinal cohorts that will more fully measure the burden of infection and serves to expand our clinical understanding of these common infections in children in the developing world.
Acknowledgments
Financial support. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Public Health Service grant AI-43596).
Potential conflicts of interest. W.A.P. has a licensing agreement for amebiasis diagnostics with TechLab; however, all royalties from this agreement are donated to the American Society of Tropical Medicine and Hygiene without benefit to W.A.P. All other authors: no conflicts.






Comments