-
PDF
- Split View
-
Views
-
Cite
Cite
M. Lindsay Grayson, Sharmila Melvani, Julian Druce, Ian G. Barr, Susan A. Ballard, Paul D. R. Johnson, Tasoula Mastorakos, Christopher Birch, Efficacy of Soap and Water and Alcohol-Based Hand-Rub Preparations against Live H1N1 Influenza Virus on the Hands of Human Volunteers, Clinical Infectious Diseases, Volume 48, Issue 3, 1 February 2009, Pages 285–291, https://doi.org/10.1086/595845
Close - Share Icon Share
Abstract
Background. Although pandemic and avian influenza are known to be transmitted via human hands, there are minimal data regarding the effectiveness of routine hand hygiene (HH) protocols against pandemic and avian influenza.
Methods. Twenty vaccinated, antibody-positive health care workers had their hands contaminated with 1 mL of 107 tissue culture infectious dose (TCID)>50/0.1 mL live human influenza A virus (H1N1; A/New Caledonia/20/99) before undertaking 1 of 5 HH protocols (no HH [control], soap and water hand washing [SW], or use of 1 of 3 alcohol-based hand rubs [61.5% ethanol gel, 70% ethanol plus 0.5% chlorhexidine solution, or 70% isopropanol plus 0.5% chlorhexidine solution]). H1N1 concentrations were assessed before and after each intervention by viral culture and real-time reverse-transcriptase polymerase chain reaction (PCR). The natural viability of H1N1 on hands for >60 min without HH was also assessed.
Results. There was an immediate reduction in culture-detectable and PCR-detectable H1N1 after brief cutaneous air drying—14 of 20 health care workers had H1N1 detected by means of culture (mean reduction, 103–4 TCID>50/0.1 mL), whereas 6 of 20 had no viable H1N1 recovered; all 20 health care workers had similar changes in PCR test results. Marked antiviral efficacy was noted for all 4 HH protocols, on the basis of culture results (14 of 14 had no culturable H1N1; P<.002) and PCR results (P<.001; cycle threshold value range, 33.3–39.4), with SW statistically superior (P<.001) to all 3 alcohol-based hand rubs, although the actual difference was only 1–100 virus copies/µL. There was minimal reduction in H1N1 after 60 min without HH.
Conclusions. HH with SW or alcohol-based hand rub is highly effective in reducing influenza A virus on human hands, although SW is the most effective intervention. Appropriate HH may be an important public health initiative to reduce pandemic and avian influenza transmission.
Although person-to-person transmission of influenza virus is due primarily to aerosol spread, transmission on the hands of patients and their caregivers is also potentially important [1–6]. Appropriate hand-hygiene (HH) practices should reduce transmission risk, but there are few in vivo data to confirm the antiviral efficacy of currently available HH protocols [7–10]. Furthermore, the long-term viability of influenza virus on unwashed human hands remains unclear, yet this has important ramifications for the risk of transmission by health care workers (HCWs) and others, should they neglect to undertake appropriate HH while caring for patients with influenza. Because of recent concerns about avian influenza and the potential for a worldwide influenza pandemic [2], we aimed to clarify theses practical issues by assessing the efficacy of various HH protocols using human volunteers cutaneously exposed to live H1N1 influenza under controlled conditions.
Methods
We assessed the virological effectiveness of 4 commonly used HH protocols with use of a standardized human study protocol, and we compared the results of these protocols with any natural change in influenza viability on HCW hands when left undisturbed by HH activities.
Influenza strain. We used a live (infectious) influenza A virus strain (A/New Caledonia/20/99 [H1N1]) that was a component of the influenza vaccine administered to Australian HCWs during 2005–2006. This H1N1 strain was considered to be a suitable surrogate for H5N1 avian influenza, because their envelopes have similar physicochemical properties, but it was less likely to be associated with a significant risk of severe illness, given the ability to prevaccinate participants [4, 6]. H1N1 was originally isolated and repassaged in embryonated chicken eggs before allantoic fluid was collected, pooled, and aliquoted into 1-mL samples that contained ∼1.8×107 tissue culture infectious dose (TCID)>50/0.1 mL live H1N1 (PCR cycle threshold [Ct] value: 17.3; World Health Organization Collaborating Centre for Influenza, Melbourne), which was stored at −70°C until used.
Participants. We recruited HCW volunteers who had undergone vaccination with the 2005 influenza vaccine (Fluvax) and had demonstrable adequate levels of antibody to influenza A before study commencement [11]. Participants were asked to avoid medicated HH products, lotions, and shampoos, as well as bathing in chlorinated pools, for 24 h before commencement of each study protocol. The hands of participants were carefully inspected, and those with dermatoses, open wounds, or other skin disorders were excluded from participation until healed. All consenting participants underwent detailed training in the correct use of each of the HH protocols and wore a fitted high-filtration (N-95) face mask, a hat, and a long-sleeved gown throughout each procedure.
All volunteers participated in the HH efficacy study, and a subset of these volunteers participated in the H1N1 viability study. Appropriate stocks of the antiviral agent oseltamivir (Tamiflu) were available to treat any participants who developed signs of clinical disease. All experiments involving humans were performed in secure negative-pressure respiratory isolation facilities at the Victorian Infectious Diseases Reference Laboratory (Melbourne, Australia), and all influenza diagnostic procedures (i.e., serological testing, PCR, and virus culture) were performed in appropriate secure laboratories at either Victorian Infectious Diseases Reference Laboratory or the World Health Organization Collaborating Centre for Influenza (Melbourne, Australia). The study was approved by the Austin Health Human Research Ethics Committee.
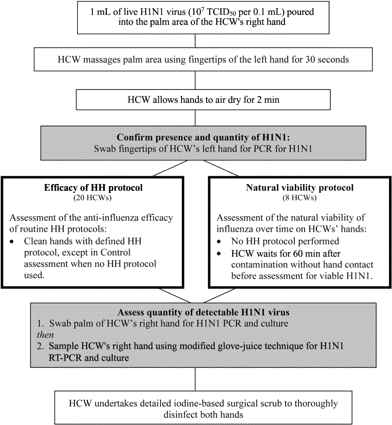
Procedure for hand contamination and virus sampling. To more accurately replicate likely clinical conditions experienced by HCWs who are exposed to large quantities of influenza-infected body fluids, we used a modified version of the American Society for Testing and Materials (ASTM) standard assessment protocols [12, 13] to contaminate HCWs' hands and used both direct swabs and a modified glove-juice technique to obtain culture specimens from their palms, fingertips, and entire hands (figure 1) [14, 15]. In brief, this involved the following: 1 mL of undiluted live H1N1 in allantoic fluid was placed in the participant's right palm, and the participant massaged it for 30 s using the fingertips of his or her left hand. This inoculum (1.8×107 TCID>50/0.1 mL) was chosen to mimic the concentrations expected in the respiratory fluids of patients with active influenza infection (usual range, 103–7 TCID>50/0.1 mL) [4, 16]. Participants then allowed their hands to air dry for 2 min before undergoing assessment to determine the presence of viable H1N1, as follows: the finger pads of all 5 digits of the left hand were swabbed using a sterile cotton-tipped applicator (Defries Industries) soaked in viral transport media, which was then stored for subsequent real-time RT-PCR (PCR) analysis.
Protocols to assess the efficacy of HH products and the natural viability of influenza virus were then followed before the quantity of detectable H1N1 was again assessed by PCR and culture (immediately after drying after each HH regimen) as follows: the palm of the participant's right hand was swabbed 10 times with a sterile cotton-tipped applicator soaked in 2 mL viral transport media, which was then stored for culture and PCR analysis. The participant's entire right hand was then also sampled by the modified glove-juice technique, with use of 5-mL viral transport media, to obtain samples for both viral culture and PCR [14, 15].
After this sampling, all participants thoroughly disinfected their hands by undertaking a supervised, detailed, surgical scrub using 7.5% povidone-iodine (Orion Laboratories) [7]. All participants were provided verbal and written information with regard to the signs and symptoms of influenza and were instructed to contact the study coordinator if any problems arose. Because the study involved the assessment of a number of HH products, each participant was required to have an interval of at least 24 h between participation in each arm of the study protocol.
PCR. Viral RNA extraction, sample spiking with internal control (bovine viral diarrheal virus [BVDV]), and reverse transcription were performed using a Magnapure automated extraction robot and random primers/AMV-RT (avian myeloblastosis virus reverse transcriptase) enzyme as described elsewhere [17]. Mastermix was prepared using ABI universal fast mastermix (Applied Biosystems) with influenza A matrix protein gene primers (forward primer, FLAM-F MGAG G T C G A A A C GTAYGTTCTCT; reverse primer, FLAM-R GTC T T G T C T T T A G CCAYTCCATGA) and probe (FLAM probe, FAM-CCC C C T C A A A G C CGA-MGB-3') in concentrations of 0.1 µmol and 0.2 µmol, respectively. For BVDV (internal control), the primers were BVDV-F 5'-TCA G C G A A G G C C G A A AAG, BVDV-R TGC T A C C C C C T C C A T T A TGC-3', and BVDV-probe VIC-CTA G C C A T G C C C T T AGT-MGB at concentrations of 0.02 µmol and 0.1 µmol, respectively. Two microliters of complementary DNA template was added to 18 µL of mastermix, and real-time PCR was performed on an ABI 7500 Fast real-time PCR system (Applied Biosystems) with use of ABI optimized reaction conditions.
The relationship between PCR cycle threshold (Ct) and influenza RNA copy number was assessed using a plasmid that contained a single copy of the matrix gene of influenza A virus. Serial log dilutions of the plasmid preparation containing 108 to 100 copies per input volume were tested in the described PCR assay, and a standard curve was constructed to enable estimation of the RNA copy number associated with the Ct value obtained from samples tested in the assay.
Viral culture. Viral culture was performed at the World Health Organization Collaborating Centre for Reference and Research on Influenza. In brief, 10-fold dilutions (10−1 to 10−8) of each test sample, including the control virus, were prepared in serum-free medium, and 100 µL was added in triplicate to wells of 96-well, flat-bottomed plates (Greiner Bio One) that contained Madin-Darby canine kidney (MDCK) cells (CCL-34, ATCC) that had been seeded a day earlier with 3×104 cells per well. After adsorption for 2 h at 35°C in 5% carbon dioxide, the inoculums were removed, and 200 µL of serum-free medium supplemented with 4 µg/mL trypsin (JRH Biosciences) was added to all wells. The plates were then incubated for 4–5 days at 35°C in 5% carbon dioxide and were observed daily, by microscopy, for cytopathic effect, and at day 4 or 5, the presence of influenza virus was confirmed by taking 25 µL of the supernatant and mixing it with 25 µL of 1% turkey RBCs, incubating at room temperature for 30 min, and assessing whether hemagglutination was present. To determine the virus titer (TCID>50/0.1 mL), the remaining medium was removed from each well and was replaced with 200 µL of 0.036% (w/v) neutral red and was incubated and washed, and residual neutral red levels were quantified by measuring the absorbance at 490 nm [18]. The TCID>50 endpoint titrations were determined using the method of Reed and Muench [19].
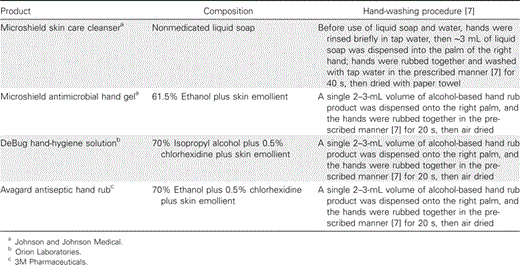
Efficacy of various HH products. After initial influenza contamination and viral confirmation assessment (figure 1), each participant used 1 of the 5 HH protocols: no HH (control), hand washing with nonmedicated liquid soap (Microshield skin care cleanser [SW]; Johnson and Johnson Medical), and hand rubbing with Microshield antimicrobial hand gel (61.5% ethanol plus skin emollient [ETOH only]; Johnson and Johnson Medical), DeBug HH solution (70% isopropyl alcohol plus 0.5% chlorhexidine plus skin emollient [ISOP-CHX]; Orion Laboratories), or Avagard antiseptic hand rub (70% ethanol plus 0.5% chlorhexidine plus skin emollient [ETOH-CHX]; 3M Pharmaceuticals). This was followed by an assessment for detectable H1N1 (table 1). The control (i.e., no HH) assessment was performed immediately after the 2-min period of air drying. Each participant performed all protocols in the same sequence (as listed in table 1), and all 4 HH products were used in the recommended manner [7], followed by assessment for detectable H1N1 and the detailed surgical scrub. All HH protocols with use of alcohol-based hand rub (ABHR) products were performed with a minimum of 24 h between each protocol.
Natural viability of influenza virus. Participants in the natural viability protocol had their hands contaminated in the usual manner, followed by culture and PCR for detectable H1N1 immediately after the 2-min period of air drying (baseline). The hands were then recontaminated and allowed to air dry for the routine 2 min, followed by a further 60-min period during which the participant simply kept his or her hands suspended in the study safety cabinet, at room temperature, without contact with any other objects or HH products. Each participant's right hand was subsequently assessed for detectable virus by PCR and culture, in the described manner, to identify any change in viral concentrations during the 60 min after initial contamination.
Statistical analysis. When appropriate, PCR results were summarized as geometric mean (±SD) values and were compared by Student's t test before and after each intervention (i.e., after use of each HH product or after the 60-min period of air drying). Because the limit of reliable PCR detection was a Ct value of 40, any specimens that had PCR Ct values >40 were arbitrarily assigned a Ct of 40.1. Results that were not normally distributed were compared using the Wilcoxon signed-rank test. A P value <.05 was considered to be statistically significant.
Results
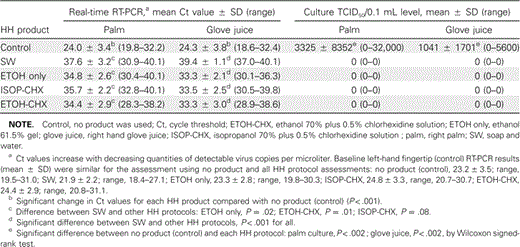
Twenty HCWs participated in the assessment of HH products, and 8 of them also participated in the natural viability study. An immediate reduction in H1N1 was noted in all participants immediately after the 2-min period of air drying, with mean (±SD) PCR Ct values of 25.2±3.9 and 25.4±3.8 for the palm and glove-juice specimens, respectively. Only 14 of 20 HCWs had culture-detectable virus in the palm and/or glove juice specimens at this baseline assessment, with a 3–4-log reduction noted, compared with the initial inoculum (107 reduced to 103–4 TCID>50/0.1 mL) (table 2). For the remaining 6 participants, no H1N1 could be detected by culture at baseline or could be reliably detected before each HH regimen.
Assessment, by PCR and culture, of the efficacy of various hand hygiene (HH) protocols against live H1N1 influenza virus on the hands of 14 human volunteers who were culture-positive at baseline.
Among the 14 participants with baseline culture-detected H1N1, marked antiviral efficacy was noted for all 4 HH protocols on the basis of both culture (14 of 14 had no culture-detected H1N1 after HH; P<.002) and PCR results (P<.001) (table 2). All HH protocols performed well, although SW hand washing appeared to be statistically superior (P<.001) to all ABHR products on the basis of PCR analysis of glove-juice fluid and to be superior to ETOH only (P=.017) and ETOH-CHX (P=.01) and comparable to ISOP-CHX (P=.08) on the basis of palm swab assessment (table 2). However, the actual difference in virus concentrations on participants' hands with SW, compared with each ABHR, was 1–100 virus copies/µL. All 3 ABHR products were found to have similar efficacy on the basis of PCR analysis of both glove juice and palm swabs (table 2), with reduction in viral contamination to ∼100 virus copies/µL. Among the 6 HCWs with baseline cultures negative for H1N1, PCR results were similar to those observed for the 14 other participants, both at baseline and after each HH protocol, which suggests that similar quantities of H1N1 were present but that virus was less viable on the skin of these individuals (data not shown).
In the assessment of natural viability of H1N1, all 8 participants had culture-detected H1N1 at baseline and had initial reductions in virus concentrations and viability after the baseline 2-min period of air drying that were similar to those found in the larger HH efficacy study. However, no further reduction in culture-detectable virus was noted after the subsequent 60-min period of noncontact air drying (not statistically significant, by Wilcoxon test). Similarly, there was no difference in the baseline-to-60-min PCR Ct values for glove-juice fluid (P=.39), but a slight decrease in virus concentrations was detected by the palm swab PCR (P=.036), which was equivalent to a reduction of <10 viral copies/µL.
Discussion
To our knowledge, this is the first human study to assess the comparative efficacy of various HH products against live influenza virus in concentrations that are likely to mimic the level of cutaneous contamination encountered during an influenza pandemic [4, 16]. We found there to be an immediate reduction in culture- and PCR-detected H1N1 virus when the virus fluid was allowed to dry on human hands, even for brief periods of only 2 min. Among the 14 participants with culture-detected H1N1 at this baseline assessment, there was a 3–4 log reduction in virus, compared with the initial inoculum. Interestingly, 6 of 20 participants repeatedly had no culture-detected virus at baseline, suggesting that in some cases, human hands may be a naturally hostile environment for H1N1 virus and that the initial act of drying and possibly the presence of natural skin oils on the hands may also have an antiviral effect. However, among the 8 HCWs who participated in the natural viability study, little change in the concentrations of H1N1 was noted after a further 60-min period during which no HH or contact was undertaken.
We found all 4 HH protocols commonly used in Australian health care settings (i.e., SW, ETOH only, ISOP-CHX, and ETOH-CHX) were highly effective in achieving a large reduction in H1N1 from human hands, with reductions to levels undetectable by culture analysis and down to ∼100 virus copies/µL by PCR analysis. Notably, SW was found to be statistically superior to all ABHR products on the basis of glove-juice analysis and superior to ethanol-containing ABHR products on the basis of direct palm swab PCR. Only isopropanol-containing ABHR was comparable to SW (P=.08) on the basis of this latter analysis. These potential slight differences in antiviral efficacy between ethanol- and isopropanol-containing products are consistent with the results of previous in vitro studies [8, 9]. Nevertheless, the actual difference in viral concentrations after SW washing, compared with concentrations after any ABHR use, was only 1–100 virus copies/µL. Given the additional time required to perform SW HH, compared with use of ABHRs, the latter may be preferred by some busy HCWs [20–22]. Also, skin emollients contained in ABHRs allow for repeated use without causing irritation [23].
Although, theoretically, some carry-over from each HH product could have technically affected the viral culture results, such carry-over should not have affected the PCR results. The fact that the decrease in PCR-detected virus was consistent with the culture results suggests that each HH regimen had a genuine impact on the quantity of H1N1 on participants' hands.
Our in vivo results are similar to the in vitro results reported by others for enveloped viruses [7–9, 24, 25] and are notable because of the known ability of influenza to survive in dust and on environmental surfaces [26–28]. Furthermore, our findings support the original 1919 observation by Lynch and Cumming [29] with regard to the potential role of hand contact on influenza transmission and are consistent with the in vivo study by Sickbert-Bennett et al. [30], who found that SW was highly effective in removing MS2 bacteriophage (a surrogate for non enveloped viruses). Similar results have also been noted in human studies of ABHRs on feline calicivirus [31]. Of course, the exact role that hand and fomite contact plays in influenza transmission in hospitals and the community has been debated [1–6]. Therefore, the importance of appropriate HH as a public health and infection-control measure remains uncertain, but it is likely to be of benefit.
Our study has some limitations. First, we would have preferred to assess a larger number of participants and to randomize the order in which each HH regimen was performed. However, given the complexity and inherent potential risks associated with the study protocol, we believe that our enrollment rate reflected the realities of conducting a study such as this. Furthermore, because our study design was a detailed surgical scrub after each HH regimen, randomizing the sequence of HH regimens is unlikely to have changed the final results. Second, the initial reduction in culture-detected H1N1 after simple 2-min air drying may be related to limitations in our detection methods rather than to any true decrease in virus viability. However, the fact that we found similar consistent changes by PCR suggests that our culture results and estimates of virus viability are likely to be valid. Third, we used a rather high contaminating concentration of H1N1 in an attempt to mimic a worst-case clinical scenario. Therefore, we cannot be absolutely certain that our results would be the same if lower H1N1 concentrations had been used—hence, our results cannot be generalized to all clinical situations. Finally, we cannot be sure whether HH products with higher concentrations of alcohol, such as those used in some European countries, would demonstrate the same or greater efficacy.
We believe that our findings have potentially important public health implications, because simple hand washing with unmedicated soap and water appears to be highly effective in removing influenza virus from hands and is, therefore, likely to be effective in preventing transmission of influenza, as long as HH is undertaken appropriately [1–3]. For busy HCWs for whom the number of HH opportunities is likely to be very high [32], the use of ABHR would seem to be a very suitable alternative. Future public health initiatives should highlight the importance of compliance with HH protocols for HCWs, patients, and caregivers.
Acknowledgments
We thank the many health care workers who volunteered their time to participate in this study. The Melbourne World Health Organization Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
Financial support. National Health & Medical Research Council (801470), Australia.
Potential conflicts of interest. M.L.G., S.M., S.A.B., and P.D.R.J. developed DeBug (as employees of Austin Health, Heidelberg, Australia), with funding in part from the Department of Human Services (Victoria, Australia). The intellectual property for this development is held by Austin Health, which handles all patent, trademark, and licensing issues. Austin Health, but no individual author, receives a small income stream from the sale of DeBug. All other authors: no conflicts.
references
Presented in part: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, September 2007 (abstract K-1796).





Comments