-
PDF
- Split View
-
Views
-
Cite
Cite
Robert C. Kaplan, Lawrence A. Kingsley, A. Richey Sharrett, Xiuhong Li, Jason Lazar, Phyllis C. Tien, Wendy J. Mack, Mardge H. Cohen, Lisa Jacobson, Stephen J. Gange, Ten-Year Predicted Coronary Heart Disease Risk in HIV-Infected Men and Women, Clinical Infectious Diseases, Volume 45, Issue 8, 15 October 2007, Pages 1074–1081, https://doi.org/10.1086/521935
Close - Share Icon Share
Abstract
Background. Highly active antiretroviral therapy (HAART), in addition to traditional vascular risk factors, may affect coronary heart disease (CHD) risk in individuals with human immunodeficiency virus (HIV) infection.
Methods. Among HIV-infected (931 men and 1455 women) and HIV-uninfected (1099 men and 576 women) adults, the predicted risk of CHD was estimated on the basis of age, sex, lipid and blood pressure levels, the presence of diabetes, and smoking status.
Results. Among HIV-infected men, 2% had moderate predicted risk of CHD (10-year CHD risk, 15%–25%), and 17% had high predicted risk (10-year CHD risk of ⩾25% or diabetes). Among HIV-infected women, 2% had moderate predicted CHD risk, and 12% had high predicted CHD risk. Compared with users of protease inhibitor–based HAART, the adjusted odds ratio (OR) for moderate-to-high risk of CHD was significantly lower among HAART-naive individuals (OR, 0.57; 95% confidence interval [CI], 0.36–0.89). Users of HAART that was not protease inhibitor based (OR, 0.74; 95% CI, 0.53–1.01) and former HAART users (OR, 0.68; 95% CI, 0.46–1.03) were also less likely than users of protease inhibitor–based HAART to have moderate-to-high CHD risk, although 95% CIs overlapped the null. Low income was associated with increased likelihood of moderate-to-high CHD risk (for annual income <$10,000 vs. >$40,000: OR, 2.32; 95% CI, 1.51–3.56 ). Elevated body mass index (calculated as weight in kilograms divided by the square of height in meters) predicted increased likelihood of moderate-to-high CHD risk (for BMI of 18.5–24.9 vs. BMI of 25–30: OR, 1.41 [95% CI, 1.03–1.93]; for BMI of 18.5–24.9 vs. BMI ⩾30: OR, 1.79 [95% CI, 1.25–2.56]).
Conclusions. Among HIV-infected adults, in addition to antiretroviral drug exposures, being overweight and having a low income level were associated with increased predicted CHD risk. This suggests a need to target HIV-infected men and women with these characteristics for vascular risk factor screening.
In the United States, use of HAART has prolonged survival dramatically among HIV-infected individuals. Therefore, the potential effects of long-term exposure to HIV infection and antiretroviral medications on cardiovascular disease (CVD) risk is of increasing concern. Several studies have reported that atherosclerosis and risk of clinical CVD events, such as myocardial infarction, may be increased with HIV infection [1–3] or use of antiretroviral drug therapy [4–7]. However, there is no evidence to suggest that CVD risks are reversing the major gains in quality and duration of life associated with HAART [8]. Nonetheless, heart disease and stroke are now among the leading causes of death in individuals with HIV infection, as they are in the general population [9, 10].
Guidelines for the management of cardiovascular risk factors among HIV-infected individuals use Framingham equation–based risk stratification for predicting each patient's likelihood of sustaining a future CVD event and for tailoring screening and treatment practices accordingly [11]. In the present study, we assessed the prevalence of major CVD risk factors and Framingham coronary heart disease (CHD) risk score among women and men participating in 2 large, comprehensive cohorts studying HIV infection in the United States: the Women's Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS). Our aims were to evaluate the 10-year predicted CHD risk among HIV-infected individuals and to identify risk factors for elevated predicted CHD risk. This would have implications for designing CVD prevention strategies for the HIV-infected population.
Methods
Study setting. The WIHS and MACS are US cohort studies involving individuals with HIV infection and HIV-uninfected control subjects. The MACS was initiated in 1984 as a study involving men who have sex with men at study locations in the metropolitan areas of Baltimore, Maryland, and Washington, DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania [12]. A total of 6973 men were enrolled during 3 time periods: 1831 HIV-positive and 3123 HIV-negative men during the period 1984–1985, 382 HIV-positive and 286 HIV-negative men during the period 1987–1990, and 689 HIV-positive and 662 HIV-negative men (primarily members of ethnic minorities) during the period 2001–2003.
The WIHS began in 1994 and has enrolled a total of 3768 women [13]. The WIHS initially enrolled 2056 HIV-positive women and 569 HIV-negative control women during the period 1994–1995 at sites in San Francisco and Los Angeles, California; Chicago, Illinois; Washington, DC; and Brooklyn and Bronx, New York. The WIHS cohort was expanded during the period 2001–2002 by 737 HIV-positive women and 406 HIV-negative control subjects. Eligibility criteria for HIV-uninfected control subjects included a history of injection drug use, a history of receipt of blood or blood products, multiple sex partners in the past 6 months, or sex with someone known to be HIV infected.
Data collection. Since the initiation of the WIHS and the MACS, all participants have been invited to complete semiannual examinations, including medical history and health-behavior questionnaires, assessments of medication use, and blood sampling. During the HAART era, the WIHS and the MACS initiated coordinated data-collection activities to investigate metabolic abnormalities and CVD. Seated blood pressure measurements were completed using a standardized protocol. Fasting laboratory values included glucose, total cholesterol, high-density lipoprotein cholesterol, triglyceride, and estimated low-density lipoprotein cholesterol levels. For individuals who did not provide fasting specimens and for fasting specimens with triglyceride levels >400 mg/dL, low-density lipoprotein cholesterol levels were measured directly.
Study participants. For the present study, we used data for HIV-infected individuals and HIV-uninfected control subjects that were collected at the most recent study visit completed from October 2003 through September 2005 (median date, May 2005). Analyses were limited to WIHS and MACS participants who did not report any history of CHD (i.e., angina, myocardial infarction, or coronary revascularization). In the MACS, of 1266 HIV-infected and 1430 HIV-uninfected men, we excluded those who had missing values for key variables (206 HIV-infected and 206 HIV-uninfected men) or who reported a history of CHD events (129 HIV-infected and 125 HIV-uninfected men). In the WIHS, of 1780 HIV-infected and 713 HIV-uninfected women, we excluded individuals who had missing values for key variables (208 HIV-infected and 91 HIV-uninfected women) or who reported a history of CHD events (117 HIV-infected and 46 HIV-uninfected women). Included in the analyses were 931 HIV-infected men, 1099 HIV-uninfected men, 1455 HIV-infected women, and 576 HIV-uninfected women.
Study variables. HAART was defined by the Department of Health and Human Services guidelines [14] as (1) ⩾2 nucleoside reverse-transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor or 1 nonnucleoside reverse-transcriptase inhibitor; (2) 1 NRTI in combination with at least 1 protease inhibitor and at least 1 nonnucleoside reverse-transcriptase inhibitor; (3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no nonnucleoside reverse-transcriptase inhibitors; or (4) an abacavir- or tenofovir-containing regimen of ⩾3 NRTIs in the absence of both protease inhibitors and nonnucleoside reverse-transcriptase inhibitors, except for the 3-NRTI regimens consisting of abacavir, tenofovir, and lamivudine or didanosine, tenofovir, and lamivudine. According to the third Adult Treatment Panel guidelines, a high low-density lipoprotein cholesterol level was defined as ⩾160 mg/dL (subjects receiving lipid-lowering therapy were also defined as having elevated low-density lipoprotein cholesterol), high triglyceride levels were defined as ⩾400 mg/dL, and low high-density lipoprotein cholesterol levels were defined as <40 mg/dL [15]. Hypertension was defined as either measured systolic blood pressure ⩾140 mm Hg, measured diastolic blood pressure ⩾90 mm Hg, or self-reported diagnosis of hypertension with use of antihypertensive medications. Diabetes was defined as either measured fasting glucose levels ⩾126 mg/dL (or measured nonfasting glucose levels ⩾200 mg/dL if fasting samples were unavailable) or self-reported diagnosis of diabetes treated with medications. With respect to body mass index (BMI; calculated as weight in kilograms divided by the square of height in meters), individuals were defined as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (⩾30.0). With respect to alcohol use, individuals were categorized as abstainers (<1 drink per week), low-to-moderate consumers (1–14 drinks per week), and heavy consumers (⩾14 drinks per week).
Statistical analysis. Analyses were stratified by age (⩾40 years of age vs. <40 years of age) because of the broad age range of study participants and the strong association of age with CHD risk. We compared HIV-infected and HIV-uninfected individuals with regard to individual CHD risk factors and used published Framingham Risk Score equations to estimate the 10-year risk of developing CHD (including myocardial infarction, CHD death, unstable angina, or chronic angina) [16]. We chose the end point of total CHD rather than “hard CHD” (defined as myocardial infarction or CHD-related death, excluding angina), because our population included young women, among whom the “hard CHD” end point is known to underestimate vascular risk (as a result of the fact that this population often presents with angina without obstructive coronary lesions) [15]. Moreover, development of incident angina in a population of young-to-middle aged adults is a clinically important end point associated with activity-limiting symptoms and adverse survival.
Risk factors used in the Framingham equations included age, sex, observed levels of metabolic risk factors (low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, and diabetes), and current smoking [16]. Predicted risk of CHD was classified as low (10-year risk, <15%), moderate (10-year risk, 15%–25%), or high (10-year risk, ⩾25%), with diabetics automatically assigned to the high-risk category [15]. Analyses limited to HIV-infected men and women were used to assess whether HIV-related and non–HIV-related factors were associated with predicted CHD risk. The OR and 95% CI describing predictors of moderate-to-high predicted CHD risk (⩾15% 10-year risk or diabetes) was obtained with multivariate logistic regression models, which included the independent variables age, sex, self-reported race and/or ethnicity, education, income, BMI, study center, alcohol consumption, history of injection drug use, cohort entry period (before 2001 or from 2001 through 2003), AIDS, and antiretroviral medication use.
Results
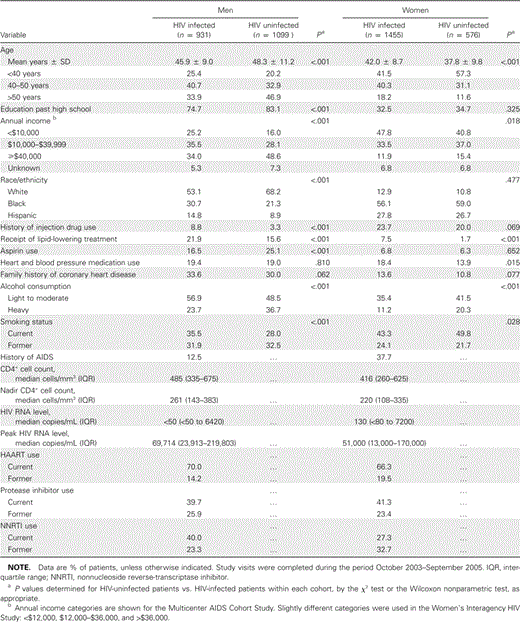
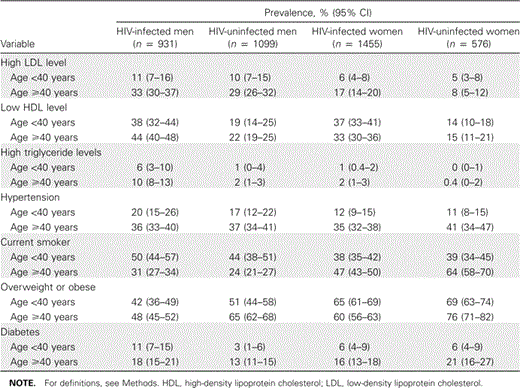
Subject characteristics. Mean age (±SD) was 45.9 ± 9.0 years among HIV-infected men, 48.3 ± 11.2 years among HIV-uninfected men, 42.0 ± 8.7 years among HIV-infected women, and 37.8 ± 9.8 years among HIV-uninfected women (table 1). Men participating in the MACS were primarily white, whereas the women participating in the WIHS were predominantly black and Hispanic. Compared with HIV-uninfected men and women, HIV-infected individuals had a higher prevalence of low high-density lipoprotein cholesterol and elevated triglyceride levels, but they were less likely to meet criteria for being overweight and obese (table 2 and figure 1). Nearly one-half of the HIV-infected men and nearly two-thirds of the HIV-infected women had BMIs in the overweight or obese range. Diabetes tended to be more prevalent among HIV-infected men than among male control subjects, but HIV-infected and HIV-uninfected women had a similar prevalence of diabetes.
Characteristics of HIV-infected and HIV-uninfected men and women in the Women's Interagency HIV Study and the Multicenter AIDS Cohort Study cohorts.
Prevalence of coronary heart disease risk factors and 10-year predicted coronary heart disease risk (Framingham equation) among HIV-infected and HIV-uninfected men and women.
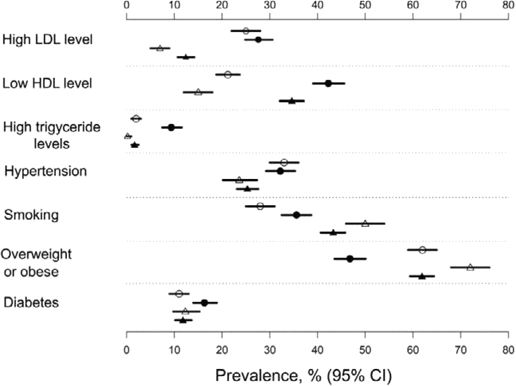
Prevalence of coronary heart disease risk factors among HIV-infected and HIV-uninfected men and women. Horizontal bars represent 95% CIs. Closed circles, HIV-infected men; closed triangles, HIV-infected women; open circles, HIV-uninfected men; open triangles, HIV-uninfected women. HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Predicted risk of CHD. Among HIV-infected men, 81% had low predicted risk of CHD (10-year risk, <15%), 2% had moderate predicted risk of CHD (10-year risk, 15%–25%), and 17% had high predicted risk of CHD (10-year risk, ⩾25% or diabetes). In comparison, among HIV-uninfected men, 5% were at moderate predicted CHD risk, and 11% were at high predicted CHD risk.
Among HIV-infected women, 86% had low predicted CHD risk, 2% had moderate predicted CHD risk, and 12% had high predicted CHD risk. In comparison, among HIV-uninfected women, 1% had moderate predicted risk, and 12% had high predicted risk.
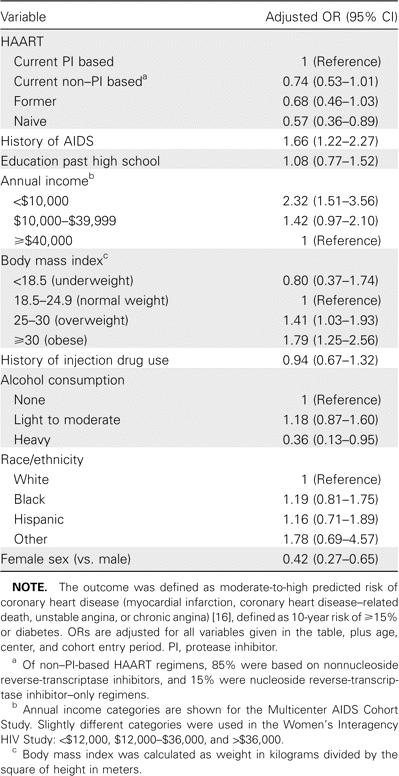
HIV-related factors associated with CHD risk. Multivariate analyses to identify characteristics associated with predicted CHD risk were limited to individuals aged ⩾40 years, because few subjects aged <40 years were at elevated 10-year predicted risk of CHD (695 of 931 HIV-infected men and 852 of 1455 HIV-infected women were aged ⩾40 years). The outcome variable was moderate-to-high predicted risk of CHD, defined as 10-year risk ⩾15% or diabetes (table 3). Among HIV-infected individuals, predicted CHD risk was lower among HAART-naive individuals than among the reference group of individuals using a protease inhibitor–based HAART regimen (adjusted OR for moderate-to-high risk, 0.57; 95% CI, 0.36–0.89). Compared with users of PI-based HAART, users of non–PI-based HAART (OR, 0.74; 95% CI, 0.53–1.01) and former HAART users (OR, 0.68; 95% CI, 0.46–1.03) also tended to be less likely to have moderate-to-high predicted CHD risk, although these comparisons did not achieve statistical significance. History of AIDS predicted a higher likelihood of having moderate-to-high CHD risk (OR, 1.66; 95% CI, 1.22–2.27).
Multivariate analyses of moderate-to-high risk of coronary heart disease among HIV-infected men and women ⩾40 years of age.
Non–HIV-related factors associated with CHD risk. Compared with subjects with an annual income >$40,000, those with an annual income <$10,000 had an OR of 2.32 (95% CI, 1.51–3.56) for moderate-to-high predicted CHD risk (table 3). Overweight and obese individuals had significantly increased predicted CHD risk. Compared with those with normal weight (BMI, 18.5–24.9), the adjusted OR for moderate-to-high CHD risk was 1.41 (95% CI, 1.03–1.93) for a BMI of 25–30 and 1.79 (95% CI, 1.25–2.56) for a BMI of ⩾30. Low income was associated with increased likelihood of moderate-to-high CHD risk. Subjects who reported heavy alcohol consumption (⩾14 drinks per week) had significantly decreased predicted CHD risk (compared with abstainers: OR, 0.36; 95% CI, 0.13–0.95). As expected, women were less likely than men to have moderate-to-high CHD predicted risk (OR, 0.42; 95% CI, 0.27–0.65).
Sensitivity analyses. In additional analyses, we estimated predicted CHD risk from the Framingham equation without automatically classifying diabetic individuals into the high-risk category [16]. Based on this approach, the median 10-year predicted risk of developing CHD was 6% (interquartile range, 4%–9%) among HIV-infected men and 2% (interquartile range, 1%–5%) among HIV-infected women. We found that 4% of HIV-infected men and 4% of HIV-infected women had moderate predicted risk (10-year risk, 15%–25%), and 1% of HIV-infected men and 0.5% of HIV-infected women had high predicted risk (10-year risk, ⩾25%).
In sex-specific analyses, similar risk factors for elevated predicted CHD risk were identified among both HIV-infected men and HIV-infected women, although 95% CIs were wide.
Discussion
We estimated the 10-year predicted risk of developing CHD (i.e., myocardial infarction, CHD death, and angina) among HIV-infected men and women without established CHD who participated in the MACS and WIHS population-based cohorts. Overall, 17% of men and 12% of women met widely-used criteria for high predicted CHD risk (a 10-year risk of CHD ⩾25% or diabetes). Many HIV-infected individuals had individual modifiable risk factors for CHD, with a high prevalence of smoking (35%–40% were current smokers), as in other cohorts of HIV-infected adults [17–21]. Over 40% of HIV-infected men and >60% of HIV-infected women met criteria for being overweight or obese (BMI ⩾25), and these individuals had increased predicted CHD risk. These findings suggest that a large fraction of the HIV-infected population would benefit from smoking cessation and weight-loss interventions.
Several earlier studies have examined predicted CHD risk among HIV-infected individuals [19–22]. Some of these can be compared directly with our data, because they included adults of similar age and used the same risk-prediction formula [16]. In a German cohort, median 10-year predicted CHD risk was estimated to be 9% among HIV-infected men (mean age, 43 years) and 2% among HIV-infected women (mean age, 37 years) [20]. In the Oslo HIV cohort study (∼80% men; mean age, 41 years), mean predicted CHD risk was 9% among HAART-treated individuals and 7% among HAART-naive individuals [19]. These results are similar to the CHD risk predictions that we estimated in the present study (men: median CHD risk, 6%; mean age, 46 years; women: median CHD risk, 2%; mean age, 42 years).
On the whole, our data are consistent with data from earlier studies that predicted a higher rate of vascular events among treated than among untreated HIV-infected adults [17, 19, 23]. In this study, compared with users of protease inhibitor–based HAART, HIV-infected subjects who were HAART-naive had significantly lower predicted risk of CHD events. Our findings may reflect adverse metabolic changes associated with protease inhibitors [24]. The fact that untreated HIV infection is associated with more favorable levels of some metabolic risk factors (e.g., reduced low-density lipoprotein cholesterol levels) may also explain the low predicted CHD risk in these individuals [25, 26]. On the other hand, it should be noted that untreated HIV infection is also associated with reduced HDL cholesterol [25, 26], which is a major adverse risk factor for vascular disease, as well as with other risk factors that are not included in the traditional CHD risk prediction models but that, nonetheless, may be important, such as hypertriglyceridemia [26] and elevations in inflammatory markers [27]. After adjustment for medication use and other factors, history of AIDS predicted increased CHD risk. This may reflect adverse metabolic changes among those with advanced disease [27]. It is also possible that AIDS may be a marker for being infected earlier in the HIV epidemic or for having less access to medical care, which may be characteristics associated with increased CHD risk.
Our findings suggest that HIV-infected individuals from economically disadvantaged populations bear a disproportionate burden of CVD risk factors. Compared with individuals with an annual income >$40,000, those with an annual income <$10,000 were more than twice as likely to have moderate-to-high predicted CHD risk. These data are consistent with the known association among non–HIV-infected populations between low socioeconomic status and increased risk of CVD [28]. Our finding that income was related to coronary risk suggests the need to target CVD screening and prevention strategies to low-income populations with HIV infection.
To date, the present study is among the largest investigations of vascular risk factors among HIV-infected women and men. However, because this was a cross-sectional study, it is not possible to conclude that associations between clinical or behavioral factors and predicted CHD risk are causal. For example, the finding of higher predicted CHD risk among abstainers than among those who consumed ⩾14 drinks per week may be attributable to bias if individuals at highest cardiac risk were more likely to receive physician advice to curtail their alcohol consumption, or it may be attributable to selective survival (i.e., early death of heavy drinkers at highest CHD risk). The Framingham risk equation has not been validated as a means to estimate future CHD risk among HIV-infected individuals. Available evidence suggests that Framingham predictions may underestimate risk among treated HIV-infected individuals [18]. Moreover, the pathophysiological mechanisms linking HIV infection with increased vascular risk may not be entirely mediated by the standard risk factors included in the Framingham risk equation. For example, abnormal coagulation or fibrinolysis [29], which are suspected risk factors for atherothrombotic events, may make a more important contribution to increased vascular risk in HIV-infected populations than in non–HIV-infected populations. HIV-infected and HIV-uninfected groups were not matched according to demographic characteristics or other factors that may affect the risk of CHD events, including use of aspirin and other CVD medications. Finally, although the WIHS and MACS are population-based cohorts, generalization of these findings should be made cautiously, because some cohort characteristics, such as the high proportion of white men in the MACS, may not reflect the current epidemic in the United States.
In summary, among HIV-infected populations, individuals using protease inhibitor–based HAART regimens had higher predicted CHD risk than did untreated individuals. Low household income was also strongly related to predicted coronary risk, suggesting that factors associated with poverty are important contributors to adverse CVD outcomes among HIV-infected individuals. Finally, intervening on risk factors (such as being overweight and smoking, which were exceedingly common) presents an opportunity to reduce the risk of vascular disease and other comorbidities, as well as to improve HIV disease progression [30].
acknowledgments
Data in this article were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group and the Multicenter AIDS Cohort Study (MACS). WIHS centers (Principal Investigators) included New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen J. Gange). MACS centers (Principal Investigators) included the Johns Hopkins University Bloomberg School of Public Health (Joseph B. Margolick, Lisa Jacobson); Howard Brown Health Center and Northwestern University Medical School (John Phair); University of California, Los Angeles (Roger Detels); and University of Pittsburgh (Charles Rinaldo).
Financial support. The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute, the National Institute of Child Health and Human Development (NICHD), the National Institute on Drug Abuse, the Agency for Health Care Policy and Research, the National Center for Research Resources, and the Centers for Disease Control and Prevention (U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-HD-32632 [NICHD], U01-AI-34993, U01-AI-42590, M01-RR00079, and M01-RR00083). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute ( UO1-AI-35042, 5-MO1-RR-00722 [General Clinical Research Centers], UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041). Additional support was provided by a grant from the National Institutes of Health and the National Heart, Lung and Blood Institute (1R01HL083760-01 to R.C.K.).
Potential conflicts of interest. All authors: no conflicts.





Comments