-
PDF
- Split View
-
Views
-
Cite
Cite
Kimberly N. Jones, Joseph C. English, Review of Common Therapeutic Options in the United States for the Treatment of Pediculosis Capitis, Clinical Infectious Diseases, Volume 36, Issue 11, 1 June 2003, Pages 1355–1361, https://doi.org/10.1086/374840
Close - Share Icon Share
Abstract
Numerous therapies are available in both over-the-counter and prescription formulations for the treatment of head lice infestation. We summarize treatment recommendations from published literature and from a recent meta-analysis from the Cochrane Database of Systematic Reviews that describe the efficacy, safety, and resistance patterns of monotherapies available in the United States. If treatment with pyrethrin or permethrin fails to eradicate the infestation, the treatment of choice is malathion. However, because of malathion's flammability, it should be a second-line therapy. Orally administered ivermectin has been reported to be efficacious, but it is not currently a US Food and Drug Administration—approved pediculicide. Alternative therapies are also discussed, in addition to indications for prophylaxis, resistance reporting, and the social impact of infestation.
Lice are 6-legged, wingless, blood-sucking insects of the order Anoplura. Each species favors a particular host, and interspecies transmission is rare [1]. Most patients carry fewer than 20 mature head lice (Pediculus humanus capitis), which survive for ∼30 days [1, 2]. The female head louse produces nearly 120 eggs (nits) that are “glued” to the hair shaft near the scalp. After 9 days, the egg hatches into a nymph (also known as an “instar” or “larva”), which then molts several times over the course of 9–15 days and becomes an adult louse [1]. In the absence of a human host, mature lice survive for 3 days and nits survive until hatching (9–10 days) [1, 2]. Fomite transmission occurs rarely [3–5]. However, head-to-head contact is the most common mode of transmission and may explain the exceptional frequency of infestation among children 3–11 years of age [1, 6–8].
Before the development of modern insecticides, various botanical treatments, inorganic poisons, and petroleum products were used to eradicate head lice. After World War II, dichlorodiphenyltrichloroethane (DDT) offered a significant advancement in treatment and continues to be used in some developing countries [1]. Because of environmental concerns and fears of increased resistance of head lice to pediculicides, other agents were developed to replace DDT, such as lindane, pyrethrin, permethrin, and malathion [9].
Cross-resistance to many pediculicides may have contributed to the recent surge in head lice infestations [1, 10]. Over-the-counter pediculicides and school-related health care expenses cost the United States >$350 million per year [1, 11. In this review, we discuss some of the most frequently used pediculicides as well as alternative approaches for the treatment of head lice. Our treatment recommendations are based on current clinical practice, published literature, and a recent meta-analysis by reviewers with the Cochrane Database of Systematic Reviews. Four of 71 randomized, placebo-controlled comparative studies met the inclusion criteria of the Cochrane meta-analysis. The criteria were as follows: (1) before the start of therapy, adult lice and nymphs must be detected plus at least 20 viable ova located ≤1.5 cm from the scalp or live lice; (2) pediculicides were not used ≤1 month preceding the trial; (3) a comb was only used for detection purposes; pediculicidal effects were evaluated via detection combing; and (4) ovicidal efficacy was determined by incubating eggs before and after pediculicidal therapy [8].
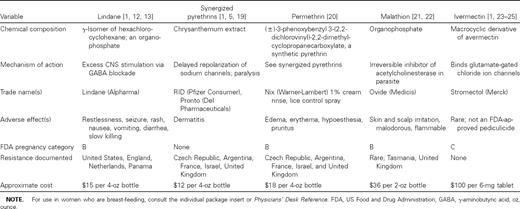
Lindane
Lindane is an organochloride with properties similar to DDT, which has potent pediculicidal and ovicidal activity [1]. Absorption of lindane through the louse exoskeleton occurs much more efficiently than through human skin [1]. As a γ-aminobutyric acid inhibitor, it causes excess CNS stimulation in and ultimate death of the ectoparasite [12, 13]. Lindane (1% shampoo) was once the primary mode of head lice eradication. However, the availability of efficacious agents with more favorable safety profiles has virtually eliminated its use for lice treatment in the United States.
Lindane resistance among head lice has been reported in the United States, the United Kingdom, the Netherlands, and Panama (table 1) [1, 14–18]. Alterations in amino acids located at the nerve sheath sodium channel receptor have been described as the most important mode of resistance and may account for observed cross-resistance to DDT, lindane, and the pyrethroids (pyrethrin and permethrin) [1].
Lindane is easily absorbed into adipose and neural tissue and has caused neurotoxicity and anemia in patients [13, 26, 27]. It is contraindicated for patients with a history of seizure, and extreme caution should be used for patients with HIV infection, who often have a reduced seizure threshold [13, 28, 29]. Minor side effects include pruritus, mild edema, burning, stinging, tingling, erythema, and rash [8].
Pyrethrin
The natural or synergized pyrethrins are a mixture of 6 active extracts from the flower heads of the ragweed relative Chrysanthemum cinerariae folium [1, 19]. Pyrethrins are usually available over the counter as a synergized formulation of 0.33% pyrethrin in 4% piperonyl butoxide. Brand names include RID (Pfizer Consumer) and Pronto (Del Pharmaceuticals). Pyrethrin blocks sodium channel repolarization of the arthropod neuron, leading to paralysis and death [5, 19]. Pyrethrins are unstable in heat and light and have no residual activity after rinsing [1].
Two applications of pyrethrins, separated by 1 week, are generally required because these agents are not ovicidal [18]. Even with appropriate application, treatment failure has occurred [19, 30]. In Panama, where baseline resistance to the natural pyrethrins is minimal, treatment efficacy ranged from only 30% (for R&C) to 52% (for RID) [31].
Combination therapy with piperonyl butoxide decreases the development of pyrethrin resistance via the mono-oxygenase pathway and greatly enhances efficacy [5, 19, 32]. Nerve sheath insensitivity, an additional mechanism of resistance, may account for the observed cross-resistance to the organochlorides (DDT and lindane) and the pyrethroids (permethrin and the natural pyrethrins) [1].
The pyrethrins have a favorable safety profile, and percutaneous absorption is minimal. They cause rare allergic side effects and should be used with caution in patients with ragweed allergies [4]. Rigorous testing, required for US Food and Drug Administration (FDA) approval, has not been performed because use of the natural pyrethrins to treat head lice was grandfathered by the US Environmental Protection Agency [1, 8, 19]. Fourteen trials (in 5, pyrethrin was administered via lotion; in 7, via shampoo; in 1, via spray; and in 1, the route of administration was unspecified) reported minor side effects, such as dry, scaly patches; edema; pruritus; and erythema. No major adverse reactions were identified [8].
Permethrin
Permethrin, (±)-3-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate, is a synthetic mixture of cis- and trans- isomers of pyrethrin chemically altered to bestow light and heat stability [1, 20, 33. It is widely used as an agricultural pesticide and insect repellent and is presently the treatment of choice for head lice infestation [1]. Permethrin acts via the same mechanism as the natural pyrethrins, causing a disruption of neuronal sodium channels that leads to paralysis and death of the parasite [5, 19, 33]. NIX (1% liquid cream rinse and a comb; Warner-Lambert) is the only FDA-approved permethrin-based pediculicide and is the only agent approved for head lice prophylaxis [5, 19, 33]. RID lice control spray (0.5% permethrin) is used to spray inanimate objects.
Currently, no adequate studies have demonstrated the superior efficacy of permethrin therapy in comparison with that of other agents [8]. However, numerous reports not accepted by Cochrane reviewers suggest that permethrin therapy is both more effective and better tolerated than is lindane therapy [34–38]. Because of its residual activity, permethrin was formerly believed to have more ovicidal activity than the natural pyrethrins do [17, 39]. Only 1 study that compared 1% permethrin cream to synergized pyrethrin met the acceptance criteria of the Cochrane reviewers [8]. It is curious that synergized pyrethrin was more ovicidal than was permethrin, a finding that illustrated the need for a second application [5, 8, 27, 40, 41].
Emerging permethrin resistance in head lice is a serious concern. In a recent study, Meinking et al. [42] reported a significantly reduced efficacy rate of current pediculicides in the United States compared with rates described only 2 years ago. Moreover, significant resistance to pyrethrin and permethrin has been noted in the Czech Republic, France, Israel, the United Kingdom, and Argentina (table 1) [1, 30, 43–46]. Several mechanisms of permethrin resistance exist, the most important of which occurs by way of the kdr (knock-down resistance) gene, which confers resistance to all concentrations of permethrin [1]. In vitro susceptibility comparisons by Pollack et al. [27] showed that lice obtained from previously treated US children exhibited significantly greater resistance in comparison with lice obtained from treatment-naive non-US children. They recommended the use of another agent if 2 treatments of permethrin failed to eradicate infestation [1, 27].
In cases of permethrin treatment failure, malathion therapy is indicated. However, 2 proposed modifications to permethrin therapy include either overnight treatment of the hair with prescription-strength permethrin 5% (Elimite; Allergan) covered by a shower cap or the extension of 1% permethrin therapy from 10 min to 30–60 min [18, 47]. Permethrin 5% (FDA-approved for scabies infestation) has the advantage of having been proven safe for children as young as 1 month of age [48].
Permethrin is the most studied pediculicide in the United States and is the least toxic to humans. Studies in which oral permethrin was administered to animals found permethrin to be 36 times less toxic than lindane is and 3 times less toxic than the natural pyrethrins are [1]. It is minimally absorbed percutaneously (<2%), compared with lindane (10%), and is rapidly degraded by mammalian esterases to the inactive form [49]. Although minor side effects, such as rash, edema, pruritus, pain, burning, stinging, tingling, possible diarrhea, and rare allergic reactions may occur, no major adverse effects have been reported [8].
Malathion
Malathion has been distributed in a 0.5% formulation as Ovide (Medicis) but has been removed from the market twice because of problems related to prolonged application time, flammability, and odor [50]. In 1999, malathion was reapproved by the FDA for the treatment of head lice. Malathion is an irreversible cholinesterase inhibitor that causes acetylcholine accumulation at the receptor site, leading to rapid death of the insect and ova [51]. Malathion binds to the sulfur atoms in the hair, which appears to offer residual protection against head lice [38]. The vehicle base contains terpenoids, which themselves offer a 50% cure rate [8, 21].
In 2 controlled in vitro susceptibility trials, malathion 0.5% lotion was the fastest-acting agent (98%–100% of lice were dead 5 min after administration) and the most effective ovicide (<5% of eggs hatched after a 10-min exposure) [1, 49, 52, 53]. Taplin et al. [21] conducted a trial accepted by Cochrane reviewers in which the ovicidal effects of malathion were compared with those of the malathion vehicle base. After a 12-h application of 0.5% malathion to the hair, 95% of patients were lice-free after 7 days and 85.5% of nits were killed [8, 21]. This is superior to the ovicidal activity of 1% permethrin, which kills 62% of eggs, as cited in a separate trial accepted by Cochrane reviewers [8, 17]. However, the ovicidal activity of the 2 agents has not been directly compared.
The manufacturer of Ovide recommends a second application 7 days after the first if lice remain [54]. However, in vitro data suggest that a single treatment time of ≤2 h may be adequate [50]. Rare malathion resistance has been observed, which occurs via acetylcholinesterase modification at the level of nerve synapses (table 1) [21, 55–57]. Malathion resistance has not been reported in the United States, and it has been useful for treating infestations of lice resistant to permethrin and pyrethrin [46, 51].
There have been reports that malathion caused Sertoli cell changes in animals, and acute toxicity in humans has only occurred in cases of agricultural exposure [51, 54, 58, 59. Active metabolites are rapidly inactivated by mammalian plasma carboxylesterases, and cholinergic crisis during the treatment of lice has not been reported. A 3-week trial of daily applications of 10% malathion dust caused insignificant cholinesterase inhibition in humans [50, 51]. Cochrane reviewers evaluated 12 studies, comprising 803 participants, in which no major adverse effects were reported [8]. Only 10% of patients experienced minor adverse reactions, which included erythema of the conjunctiva, sclera, and scalp; scalp tingling; and dandruff [8]. The safety of malathion has not been established for the treatment of children <2 years old [51]. In addition, it is important to note that the alcohol base of malathion is very flammable, and patients should be instructed to avoid the use of hair dryers or curling irons during treatment [54].
Ivermectin
Ivermectin is a macrocyclic lactone that is structurally similar to macrolide antibiotics, although it lacks antibacterial activity [1]. Avermectin, from which ivermectin is derived, was isolated from Streptomyces avermitilis in the 1970s and has powerful antihelminthic activity [1, 60]. Ivermectin binds with high affinity to glutamate-gated chloride channels in invertebrate muscle and nerve cells [23]. Free movement of chloride through opened chloride ion channels results in hyperpolarization with secondary paralysis and eventual death [23]. It is indicated for the treatment of onchocerciasis and strongyloidiasis but has not been approved by the FDA for the treatment of lice infestation [23, 47]. Nonetheless, off-label use has demonstrated that a regimen of 2 doses of oral ivermectin (200 µg/kg) separated by 10 days has been effective [18, 24, 25]. No instances of resistance to ivermectin among head lice have been reported to date [1, 23, 61]. In addition, no serious side effects have been reported.
Trimethoprim-Sulfamethoxazole (Tmp-Smx)
TMP-SMX (Watson) has been used to treat eyelid infestation with Pediculosis pubis [1]. Studies suggest that TMP-SMX effectively treats head lice infestation if provided for 3 days and followed by another 3-day course after a 7–10-day interval [47, 62]. Elimination of bacteria, needed by head lice for the production of B vitamins and nit glue, is presumed to be the primary mode of action of TMP-SMX [1]. In a randomized controlled trial, Hipolito and others [63] recently compared 1% permethrin cream, a 10-day course of oral TMP-SMX, and a combination of both treatments. The efficacy rates of the 3 treatments were 79%, 83%, and 95%, respectively. As a result of rare but severe side effects, such as toxic epidermal necrolysis, Stevens-Johnson syndrome, aplastic anemia, and blood dyscrasias, treatment with TMP-SMX should be reserved for severe infestations or subsequent bacterial superinfections [1].
Other Therapies
Crotamiton has been used rarely for cases of head lice infestation [39, 41, 64]. Although the mechanism of action is unknown, it appears to offer exceptional antipruritic activity [5]. Preliminary reports suggest that 2 consecutive nighttime applications safely eradicates lice from adults [5]. The safety of crotamiton for treating children, however, has not been established [19].
Levamisole (3.5 mg/kg for 10 days; Janssen) eradicated head lice in 85% of patients in one small study. There were no adverse effects noted. However, this was a small trial that included only 28 children [65].
Hair Clean 1-2-3 (Quantum), a product that contains anise, ylang-ylang, coconut oils, and isopropyl alcohol, is sold in some health stores. Although the mechanism of action is unknown, this product was found to be more efficacious than 1% permethrin after 2 weekly treatments (98% vs. 89%) in a nonrandomized study by Meinking [1]. Because only limited data are available, the National Pediculosis Association currently does not recommend the use of essential oils for the treatment of head lice infestation [66].
Some easily attained items, such as mayonnaise, petroleum jelly, and Dippity-Do hair styling gel, occlude lice spiracles and effectively decrease respiratory exchange [1, 41]. These products can be applied at night to the hair, which is then covered with a shower cap [47]. Overall, these oils have been shown to slow the movement of lice, making their removal with a comb easier. However, these products show little killing activity compared with commercial pediculicidal products [1]. Petroleum jelly can be removed by shampooing with Pert (Procter & Gamble; D. Elston, personal communication).
Use of nit combs is important for increasing the efficacy of treatment and to allow children to reenter schools whose policies require the child to be “nit-free” before returning [41]. Vinegar or formic acid (8%) may enhance nit removal by softening nit glue [67]. A 10-min application to the hair, followed by rinsing with water, drying, and then combing, is recommended [68]. Compared with insecticide therapy alone, which only removes up to 27.6% of nits, treatment with formic acid and a pediculicide removed 93.5% in a nonrandomized controlled study [68].
Conclusion
In the treatment of lice and other infestations, it is important to consider efficacy, safety, expense, availability, patient preference, and ease of application (table 1). Assessment by the clinician of the severity of the infestation, the number of recurrences, the local level of resistance, and the potential for transmission is also critical.
Many studies suggest that permethrin is superior to synergized pyrethrins and that pyrethrins are superior to lindane in the treatment of head lice infestation. Only 4 of 71 trials that compared the efficacy of insecticides met the acceptance criteria designated by Cochrane reviewers, and 3 trials revealed no significant therapeutic difference between malathion, permethrin, and synergized pyrethrin [8, 17, 21, 40, 69]. Unfortunately, these studies were conducted in developing countries among patients who had no previous exposure to pediculicides, a population not reflective of infested patients in the United States. Some studies as well as the US Centers for Disease Control and Prevention (CDC) suggest that malathion has superior pediculicidal and ovicidal activity, making this agent an attractive therapeutic option for patients infested with drug-resistant lice [49, 52, 70]. However, careful comparative studies are needed to confirm its superior efficacy. Unlike permethrin, malathion is not approved by the FDA for the treatment of infants ≤2 months of age.
With the emergence of drug resistance among head lice, modifications to present treatment regimens and combination therapies, such as permethrin with oral ivermectin, may be more efficacious until more effective agents are developed. In addition, use of a nit comb remains a critical factor in complete lice and egg removal, and formic acid may provide additional treatment effectiveness. Although the occurrence of transmission by fomites is controversial, most experts recommend washing bedding and clothing in hot water [41].
Treating contacts (e.g., classmates, nursing home patients, and other institutional contacts) may be an important factor in successful head lice therapy and for reducing the development of resistance [41]. Results from an 18-year study by Meinking et al. [1] suggest that prophylactic treatment of close contacts of infested children offers more benefit than repeated treatment of one child [39]. Nonetheless, epidemic infestations are rare and use of prophylaxis is controversial [71]. Although the National Pediculosis Association is presently evaluating prophylactic treatment against head lice infestation, current opinion discourages the use of prophylaxis because this may expedite already rapidly emerging resistance [70].
Cohabitants of infested individuals should be examined carefully and treated simultaneously only if there is evidence of ongoing infestation. Children should be excused from school only if there is evidence of active infection [71]. Indications for adults are not clearly defined because it seems unlikely that transmission would occur in a contact-free work environment, especially after an overnight treatment. Cases in which resistance is suspected should be reported to the National Pediculosis Association, where data is compiled and eventually forwarded to the CDC [66]. The appropriate state health department should also be notified.





Comments