-
PDF
- Split View
-
Views
-
Cite
Cite
Manish Sadarangani, David W. Scheifele, Scott A. Halperin, Wendy Vaudry, Nicole Le Saux, Raymond Tsang, Julie A. Bettinger, N. Bridger, R. Morris, S. Halperin, K. Top, P. Déry, D. Moore, M. Lebel, N. Le Saux, D. Tran, L. Ford-Jones, J. Embree, B. Law, R. Tsang, B. Tan, W. Vaudry, T. Jadavji, O. G. Vanderkooi, D. Scheifele, L. Sauvé, J. Bettinger, For the investigators of the Canadian Immunization Monitoring Program, ACTive (IMPACT), The Impact of the Meningococcal Serogroup C Conjugate Vaccine in Canada Between 2002 and 2012, Clinical Infectious Diseases, Volume 59, Issue 9, 1 November 2014, Pages 1208–1215, https://doi.org/10.1093/cid/ciu597
Close - Share Icon Share
Abstract
Background. Before 2001, the incidence of invasive meningococcal disease (IMD) in Canada was 1.0 per 100 000 per year, with 40% of cases caused by serogroup C organisms. During 2001–2005 all provinces introduced the meningococcal serogroup C conjugate vaccine (MCCV) into their routine infant immunization schedule.
Methods. Active, prospective, population-based surveillance of IMD in children and adults was conducted by the Canadian Immunization Monitoring Program, ACTive (IMPACT) during 2002–2012. Inclusion criteria were admission to hospital and identification of Neisseria meningitidis from a sterile site. Incidence was estimated using population census data from Statistics Canada.
Results. Prior to MCCV introduction, serogroup C disease incidence was 0.07–0.25 per 100 000 per year depending on the province. Following vaccine introduction, serogroup C disease decreased to <0.05 per 100 000 per year, with a reduction of 14% per year (P = .0014). A decrease occurred in all provinces, despite differing schedules being implemented. The largest decrease of 83% (from 0.27 to 0.05 per 100 000 per year) occurred in the 15–24 year age group (P = .0100) who were not vaccinated in all provinces. There was no impact on the incidence of nonserogroup C disease over the same period (P = .9811).
Conclusions. MCCV dramatically reduced the incidence of serogroup C IMD in Canada through both direct and indirect effects. The observation that disease incidence decreased with different schedules suggests that the doses at 12 months (common to all provinces) and adolescence (7 of 8 provinces studied) were critical in achieving disease control.
(See the Major Article by Bijlsma et al on pages 1216–21, and the Editorial Commentary by Maiden and MacLennan on pages 1222–4.)
Neisseria meningitidis causes around 500 000 cases of meningitis and septicemia globally every year and has a case-fatality rate of 10% in developed countries [1]. The organism is carried asymptomatically in the nasopharynx by approximately 10% of the population, with highest carriage rates of up to 25% in 15–25 year-olds [2, 3]. The peak incidence of invasive disease occurs in children between 6 months and 2 years of age, with a second smaller peak in adolescents and young adults. In Canada, the incidence of invasive meningococcal disease (IMD) has been <2.1 cases per 100 000 per year since the 1950s, with the majority of disease being caused by serogroup B and C organisms [4]. Between 1995 and 2001 the incidence was just below 1.0 per 100 000 per year, with 40% of cases caused by serogroup C. There was a rapid increase in serogroup C incidence between 1998 and 2001, when it reached almost 0.6 per 100 000 per year and caused over half of all cases [4]. In addition, there were 8 outbreaks of serogroup C disease in Canada between 1999 and 2001 [5].
In October 2001, the National Advisory Committee on Immunization (NACI) recommended inclusion of the meningococcal C conjugate vaccine (MCCV) in the routine childhood immunization schedule in Canada [6]. The initial recommendation was for 3 doses at 2, 4, and 6 months of age, with a modified schedule for older children [6, 7]. Current schedules include 0–3 doses in infants (<1 year of age), 1 dose at 12–23 months, and 1 dose at 12–24 years. Between 2001 and 2005, all provinces and territories except Nunavut introduced MCCV into the routine schedule. Differences in local disease epidemiology and programmatic considerations led to a variety of schedules being used in different regions of Canada.
The primary objective of this study was to determine the incidence of serogroup C IMD before and after introduction of MCCV across Canada. Secondary objectives were to assess the impact on other serogroups, and in particular examine if there were any evidence of serogroup replacement, as well as to use the provincial variability in immunization schedules as an opportunity to compare different dosing schedules, and to compare the impact of the vaccine in different age groups, including any difference between those who were targeted for vaccination (direct effects) and those who were not (indirect effects).
PATIENTS AND METHODS
Study Locations
Active, prospective, population-based surveillance of IMD in children and adults across Canada has been conducted by the Canadian Immunization Monitoring Program, ACTive (IMPACT) since 2002. Surveillance was coordinated by 12 urban centers, which collected data from over 150 hospitals in 8 provinces. The catchment area of these hospitals included over 16 million individuals, around 50% of the Canadian population, and approximately 90% of the pediatric tertiary care beds in Canada. This study included patients admitted to hospital with IMD between 1 January 2002 and 31 December 2012. To enable incidence rate calculations, a defined study population area was created for each IMPACT center based on the catchment areas of study hospitals.
Provinces used different schedules when implementing the MCCV Program (Supplementary Table 1). In Quebec MCCV was introduced in 2001 with a campaign to vaccinate all those between 2 months and 20 years of age, followed by routine immunization of 12-month old children and catch-up for older children. Alberta introduced the vaccine with doses at 2, 4, and 6 months from 2002 (and modified to 2, 4, and 12 months in 2007) with the addition of an adolescent dose at 14–16 years in 2010. In British Columbia, the vaccine was introduced in 2003 with doses at 12 months and 11–12 years. An additional dose at 2 months was added in 2005 and a catch-up program was offered to adolescents aged 14–18 years during 2004–2007. In 2004 Ontario and Saskatchewan introduced the vaccine, followed by Nova Scotia, Manitoba and Newfoundland and Labrador in 2005. These 5 provinces all introduced a 2-dose schedule, with an initial dose at 12 months and a booster/catch-up dose between 10 and 16 years, depending on the province. Manitoba initially introduced the adolescent dose only, changing to a 2-dose schedule in 2009. In some provinces the adolescent dose is now administered as a component of the meningococcal quadrivalent conjugate vaccine, which protects against serogroups A, C, W, and Y.
Study Subjects
Patients with N. meningitidis identified by culture or polymerase chain reaction from a normally sterile body fluid or tissue, most commonly blood and/or cerebrospinal fluid, were included. Cases were actively identified via microbiology laboratories, infection control practitioners, ward and intensive care unit staff and local public health units, and by interrogation of hospital databases for relevant discharge codes based on International Classification of Diseases (ICD)-9 and ICD-10, which included terms for meningococcal disease.
Data Collection
Clinical data were collected from patient health records, and immunization history was also collected from immunization registries and family doctors. All information was recorded into a standard form, which was reviewed at the IMPACT data center before being entered into an electronic database. A dual data entry process was used with separate operators and preprogrammed consistency checks.
Characterization of Bacterial Isolates
All isolates were characterized initially in the local and provincial laboratories according to standard procedures and then sent to the National Microbiology Laboratory where the serogroup was confirmed and isolates were stored.
Incidence Rate Analysis
Age-specific population estimates of the defined study population areas were obtained from the 2006 Census of Population [8]. Study subjects residing outside the defined study population area were excluded from the incidence analysis. Poisson regression models were used to examine trends in the incidence of IMD, categorized by serogroup, province, age, and immunization status, and the logarithm of the population was used as an offset for the Poisson regression. The incidence rate ratio (IRR) was calculated for each model, to estimate the relative change in incidence per year and determine P-values. SAS (version 9.3, SAS Institute, Cary, NC) was used for all analyses.
For each province the year of introduction of MCCV was considered to be year 0 of the MCCV Program, with years prior to vaccine introduction designated −3 to −1 and years following MCCV +1 to +11. Data from all IMPACT provinces contributed to years +1 to +7, whereas years −3 to −1 included data from all IMPACT provinces except Quebec and Alberta and data for years +8 to +11 were based on Quebec, Alberta, British Columbia, Ontario, and Saskatchewan (Table 1). Analysis of serogroup-specific incidence rates was performed for years −2 to +8 as these years included the majority of the study population. This avoided distortion of the data by local epidemiologic variations, such as an ongoing outbreak of serogroup B disease in Quebec [9]. Analysis for direct effect of the vaccine included all individuals within age groups who were targeted for vaccination (Supplementary Table 1). All other individuals were included in the analysis of indirect effects of the vaccine. Alberta was excluded from the analysis of indirect effects as the adolescent dose was only introduced in 2010, and indirect effects are mediated via interruption of transmission in this age group. For Manitoba, children aged 1–4 years were excluded from the analysis of direct and indirect effects as this group would have been classified in the indirect group initially, and then the direct group from 2009 following introduction of the dose in early childhood.
Number of Cases of Serogroup C and all IMD Each Year by Province During 2002–2012, by Year of Meningococcal Serogroup C Conjugate Vaccine (MCCV) Program
| Province . | Populationa . | Number of Cases of Serogroup C/Number of Cases of all IMD per Year of MCCV Program in Each Provinceb (Calendar Year) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −3 . | −2 . | −1 . | Year 0 . | +1 . | +2 . | +3 . | +4 . | +5 . | +6 . | +7 . | +8 . | +9 . | +10 . | +11 . | Total . | ||
| QC | 2 512 669 | … | … | … | … | 10/23 | 5/21 | 4/24 | 1/21 | 3/21 | 4/28 | 3/21 | 0/20 | 0/26 | 1/40 | 0/28 | 31/273 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| AB | 2 280 379 | … | … | … | 14/19 | 4/10 | 4/11 | 1/5 | 0/5 | 0/11 | 1/9 | 0/6 | 0/5 | 1/10 | 5/8 | … | 30/99 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| BC | 2 549 714 | … | … | 6/21 | 2/13 | 10/16 | 6/15 | 2/12 | 6/22 | 4/9 | 1/14 | 1/5 | 0/8 | 0/5 | … | … | 38/140 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| ON | 7 156 082 | … | 5/19 | 5/23 | 2/20 | 5/14 | 8/26 | 7/30 | 1/24 | 6/23 | 2/11 | 1/8 | 1/6 | … | … | … | 43/204 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| SK | 596 539 | … | 0/0 | 1/1 | 0/1 | 0/2 | 0/2 | 0/3 | 0/2 | 0/2 | 0/0 | 0/1 | 0/1 | … | … | … | 1/15 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| MB | 654 953 | 2/7 | 1/3 | 0/4 | 0/0 | 0/1 | 0/3 | 1/5 | 0/0 | 2/6 | 0/2 | 0/1 | … | … | … | … | 6/32 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NS | 404 746 | 0/3 | 1/1 | 1/6 | 1/1 | 0/0 | 0/1 | 4/7 | 0/2 | 0/1 | 0/2 | 0/1 | … | … | … | … | 7/25 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NL | 297 026 | 0/3 | 1/2 | 0/0 | 0/0 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 0/0 | 0/0 | … | … | … | … | 1/19 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| Total | 16 452 108 | 2/13 | 8/25 | 13/55 | 19/54 | 29/69 | 23/82 | 19/89 | 8/79 | 15/75 | 8/66 | 5/43 | 1/40 | 1/41 | 6/48 | 0/28 | 157/807 |
| Province . | Populationa . | Number of Cases of Serogroup C/Number of Cases of all IMD per Year of MCCV Program in Each Provinceb (Calendar Year) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −3 . | −2 . | −1 . | Year 0 . | +1 . | +2 . | +3 . | +4 . | +5 . | +6 . | +7 . | +8 . | +9 . | +10 . | +11 . | Total . | ||
| QC | 2 512 669 | … | … | … | … | 10/23 | 5/21 | 4/24 | 1/21 | 3/21 | 4/28 | 3/21 | 0/20 | 0/26 | 1/40 | 0/28 | 31/273 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| AB | 2 280 379 | … | … | … | 14/19 | 4/10 | 4/11 | 1/5 | 0/5 | 0/11 | 1/9 | 0/6 | 0/5 | 1/10 | 5/8 | … | 30/99 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| BC | 2 549 714 | … | … | 6/21 | 2/13 | 10/16 | 6/15 | 2/12 | 6/22 | 4/9 | 1/14 | 1/5 | 0/8 | 0/5 | … | … | 38/140 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| ON | 7 156 082 | … | 5/19 | 5/23 | 2/20 | 5/14 | 8/26 | 7/30 | 1/24 | 6/23 | 2/11 | 1/8 | 1/6 | … | … | … | 43/204 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| SK | 596 539 | … | 0/0 | 1/1 | 0/1 | 0/2 | 0/2 | 0/3 | 0/2 | 0/2 | 0/0 | 0/1 | 0/1 | … | … | … | 1/15 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| MB | 654 953 | 2/7 | 1/3 | 0/4 | 0/0 | 0/1 | 0/3 | 1/5 | 0/0 | 2/6 | 0/2 | 0/1 | … | … | … | … | 6/32 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NS | 404 746 | 0/3 | 1/1 | 1/6 | 1/1 | 0/0 | 0/1 | 4/7 | 0/2 | 0/1 | 0/2 | 0/1 | … | … | … | … | 7/25 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NL | 297 026 | 0/3 | 1/2 | 0/0 | 0/0 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 0/0 | 0/0 | … | … | … | … | 1/19 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| Total | 16 452 108 | 2/13 | 8/25 | 13/55 | 19/54 | 29/69 | 23/82 | 19/89 | 8/79 | 15/75 | 8/66 | 5/43 | 1/40 | 1/41 | 6/48 | 0/28 | 157/807 |
Abbreviations: AB, Alberta; BC, British Columbia; IMD, invasive meningococcal disease; MB, Manitoba; MCCV, meningococcal serogroup C conjugate vaccine; NL, Newfoundland and Labrador; NS, Nova Scotia; ON, Ontario; QC, Quebec; SK, Saskatchewan.
a Population figures represent the total population in the defined study population area in each province based on the 2006 Census of Population carried out by Statistics Canada (available at: http://www12.statcan.gc.ca/census-recensement/2006/index-eng.cfm).
b Year of introduction of MCCV was considered to be ‘Year 0,’ with years prior to vaccine introduction designated −3 to −1 and years following MCCV +1 to +11, depending on the province (see Supplementary Table 1 for full details of vaccine introduction in each province).
Number of Cases of Serogroup C and all IMD Each Year by Province During 2002–2012, by Year of Meningococcal Serogroup C Conjugate Vaccine (MCCV) Program
| Province . | Populationa . | Number of Cases of Serogroup C/Number of Cases of all IMD per Year of MCCV Program in Each Provinceb (Calendar Year) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −3 . | −2 . | −1 . | Year 0 . | +1 . | +2 . | +3 . | +4 . | +5 . | +6 . | +7 . | +8 . | +9 . | +10 . | +11 . | Total . | ||
| QC | 2 512 669 | … | … | … | … | 10/23 | 5/21 | 4/24 | 1/21 | 3/21 | 4/28 | 3/21 | 0/20 | 0/26 | 1/40 | 0/28 | 31/273 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| AB | 2 280 379 | … | … | … | 14/19 | 4/10 | 4/11 | 1/5 | 0/5 | 0/11 | 1/9 | 0/6 | 0/5 | 1/10 | 5/8 | … | 30/99 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| BC | 2 549 714 | … | … | 6/21 | 2/13 | 10/16 | 6/15 | 2/12 | 6/22 | 4/9 | 1/14 | 1/5 | 0/8 | 0/5 | … | … | 38/140 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| ON | 7 156 082 | … | 5/19 | 5/23 | 2/20 | 5/14 | 8/26 | 7/30 | 1/24 | 6/23 | 2/11 | 1/8 | 1/6 | … | … | … | 43/204 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| SK | 596 539 | … | 0/0 | 1/1 | 0/1 | 0/2 | 0/2 | 0/3 | 0/2 | 0/2 | 0/0 | 0/1 | 0/1 | … | … | … | 1/15 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| MB | 654 953 | 2/7 | 1/3 | 0/4 | 0/0 | 0/1 | 0/3 | 1/5 | 0/0 | 2/6 | 0/2 | 0/1 | … | … | … | … | 6/32 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NS | 404 746 | 0/3 | 1/1 | 1/6 | 1/1 | 0/0 | 0/1 | 4/7 | 0/2 | 0/1 | 0/2 | 0/1 | … | … | … | … | 7/25 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NL | 297 026 | 0/3 | 1/2 | 0/0 | 0/0 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 0/0 | 0/0 | … | … | … | … | 1/19 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| Total | 16 452 108 | 2/13 | 8/25 | 13/55 | 19/54 | 29/69 | 23/82 | 19/89 | 8/79 | 15/75 | 8/66 | 5/43 | 1/40 | 1/41 | 6/48 | 0/28 | 157/807 |
| Province . | Populationa . | Number of Cases of Serogroup C/Number of Cases of all IMD per Year of MCCV Program in Each Provinceb (Calendar Year) . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −3 . | −2 . | −1 . | Year 0 . | +1 . | +2 . | +3 . | +4 . | +5 . | +6 . | +7 . | +8 . | +9 . | +10 . | +11 . | Total . | ||
| QC | 2 512 669 | … | … | … | … | 10/23 | 5/21 | 4/24 | 1/21 | 3/21 | 4/28 | 3/21 | 0/20 | 0/26 | 1/40 | 0/28 | 31/273 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| AB | 2 280 379 | … | … | … | 14/19 | 4/10 | 4/11 | 1/5 | 0/5 | 0/11 | 1/9 | 0/6 | 0/5 | 1/10 | 5/8 | … | 30/99 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| BC | 2 549 714 | … | … | 6/21 | 2/13 | 10/16 | 6/15 | 2/12 | 6/22 | 4/9 | 1/14 | 1/5 | 0/8 | 0/5 | … | … | 38/140 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| ON | 7 156 082 | … | 5/19 | 5/23 | 2/20 | 5/14 | 8/26 | 7/30 | 1/24 | 6/23 | 2/11 | 1/8 | 1/6 | … | … | … | 43/204 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| SK | 596 539 | … | 0/0 | 1/1 | 0/1 | 0/2 | 0/2 | 0/3 | 0/2 | 0/2 | 0/0 | 0/1 | 0/1 | … | … | … | 1/15 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| MB | 654 953 | 2/7 | 1/3 | 0/4 | 0/0 | 0/1 | 0/3 | 1/5 | 0/0 | 2/6 | 0/2 | 0/1 | … | … | … | … | 6/32 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NS | 404 746 | 0/3 | 1/1 | 1/6 | 1/1 | 0/0 | 0/1 | 4/7 | 0/2 | 0/1 | 0/2 | 0/1 | … | … | … | … | 7/25 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| NL | 297 026 | 0/3 | 1/2 | 0/0 | 0/0 | 0/3 | 0/3 | 0/3 | 0/3 | 0/2 | 0/0 | 0/0 | … | … | … | … | 1/19 |
| ('02) | ('03) | ('04) | ('05) | ('06) | ('07) | ('08) | ('09) | ('10) | ('11) | ('12) | |||||||
| Total | 16 452 108 | 2/13 | 8/25 | 13/55 | 19/54 | 29/69 | 23/82 | 19/89 | 8/79 | 15/75 | 8/66 | 5/43 | 1/40 | 1/41 | 6/48 | 0/28 | 157/807 |
Abbreviations: AB, Alberta; BC, British Columbia; IMD, invasive meningococcal disease; MB, Manitoba; MCCV, meningococcal serogroup C conjugate vaccine; NL, Newfoundland and Labrador; NS, Nova Scotia; ON, Ontario; QC, Quebec; SK, Saskatchewan.
a Population figures represent the total population in the defined study population area in each province based on the 2006 Census of Population carried out by Statistics Canada (available at: http://www12.statcan.gc.ca/census-recensement/2006/index-eng.cfm).
b Year of introduction of MCCV was considered to be ‘Year 0,’ with years prior to vaccine introduction designated −3 to −1 and years following MCCV +1 to +11, depending on the province (see Supplementary Table 1 for full details of vaccine introduction in each province).
Ethical Considerations
Appropriate approvals for the study were obtained in all hospitals.
RESULTS
Population Characteristics
There were 943 cases of IMD in the study population between 2002 and 2012 and 81 deaths, giving a case-fatality rate of 8.6%. For the 172 serogroup C disease cases (18% of the total), the average age was 33 years, and the case-fatality rate was 12.2%. Within the defined study population area there were 807 cases, and these were included in further analyses. There were a total of 157 cases of serogroup C disease in the defined study population area (19% of the total), with a peak of 37 cases in 2002 and fewer than 8 cases per year from 2009 onward (Table 1).
Serogroup-Specific Incidence of IMD in Canada
Between 2002 and 2005, during the introduction of MCCV across Canada, the incidence of IMD was 0.14 per 100 000 per year for serogroup C and 0.33 per 100 000 per year for all other serogroups combined. Between 2009 and 2012, when all vaccine programs had been established, the incidence had decreased by 77% to 0.03 per 100 000 per year for serogroup C (P < .0001), with no significant change in non-C disease, which was 0.34 per 100 000 per year (P = .9811). Separate analyses for serogroups B and Y confirmed there was no significant change in the incidence of these serogroups (P = .5676 and P = .1768, respectively). During this time serogroup C decreased from 30% of all IMD in 2002–2005 to 8.5% in 2009–2012.
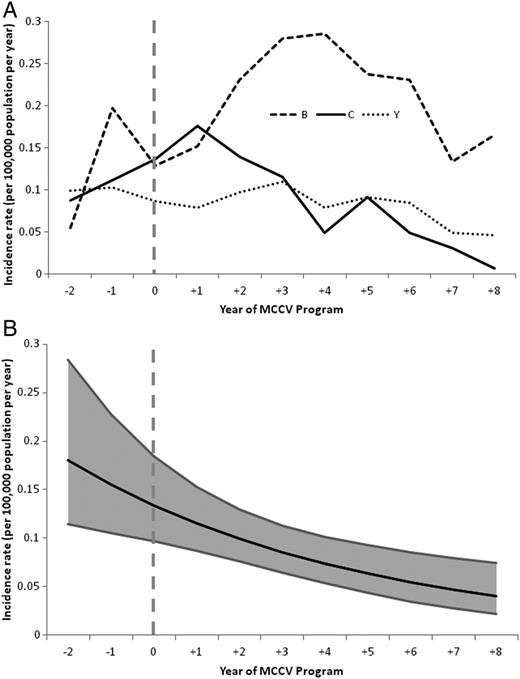
In relation to year of MCCV introduction, the incidence of serogroup C disease was 0.10 per 100 000 per year during years −2 to −1, with the highest rates in Nova Scotia and British Columbia (0.25 and 0.24 per 100 000 per year, respectively). In the 8 years following vaccine introduction, serogroup C disease decreased to <0.05 per 100 000 per year, declining at a rate of 14% per year (P = .0014) (Figure 1).
A, Serogroup-specific incidence rates of IMD from year −2 to +8 of MCCV Program in all provinces. B, Poisson regression model of incidence of serogroup C IMD from year −2 to +8 of MCCV Program in all provinces. Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
Incidence of Serogroup C IMD in Different Provinces
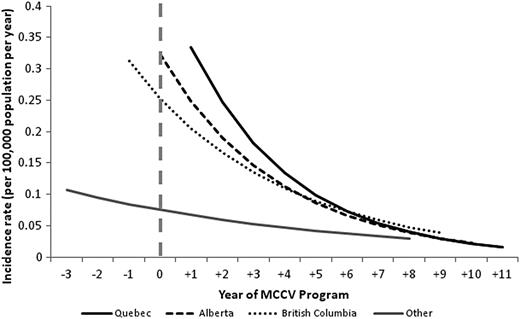
There was a similar reduction of serogroup C disease in all provinces (Figure 2). In Quebec, there was a 98% reduction in serogroup C disease between year +1 and years +8 to +11 (P < .0001). In Alberta, the incidence was 0.61 per 100 000 per year in 2002 (year 0), and this decreased by 98% to 0.01 per 100 000 between years +4 and +9 (P = .0051). However, there were 5 serogroup C cases in 2012 (year +10) in unvaccinated individuals in Alberta, and when these were included in the analysis, the overall decrease of 93% observed throughout the study period no longer achieved statistical significance (P = .1195). Four of these cases occurred in individuals aged between 11 years and 23 years, with 1 additional case in a 40-year old. British Columbia had a significant reduction of 92% in serogroup C disease (P = .0088), and in provinces using a 2-dose schedule there was a downward trend, with a decrease of 66% from years −3 to −1 until +4 to +8 (P = .0825).
Poisson regression models of incidence rates of serogroup C IMD from year −3 to +11 of MCCV Program by province. Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
Age-Specific Incidence Rates of Serogroup C IMD
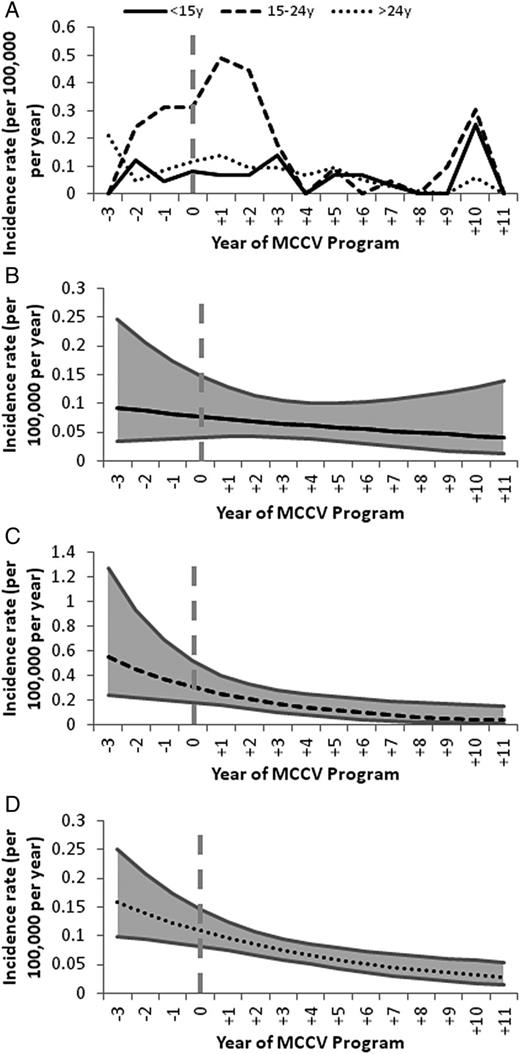
The highest incidence of serogroup C IMD occurred in the 15–24 year age group (Figure 3) and declined by 83% from 0.27 per 100 000 per year (years −3 to −1) to 0.05 per 100 000 per year (years +4 to +11; P = .0100). Although the overall incidence in adults aged over 24 years was lower, there was a significant reduction in serogroup C disease of 41% (P = .0009). In children <15 years of age there was a downward trend in incidence, with a drop of 44% (P = .4284).
A, Incidence rates of serogroup C IMD from year −3 to +11 of MCCV Program in all provinces, by age group. B–D, Poisson regression models of incidence of serogroup C IMD from year −3 to +11 of MCCV Program in all provinces in children <15 years (B) adolescents and young adults 15–24 years (C) and adults >24 years (D). Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
Comparison of Direct and Indirect Effects of the Vaccine
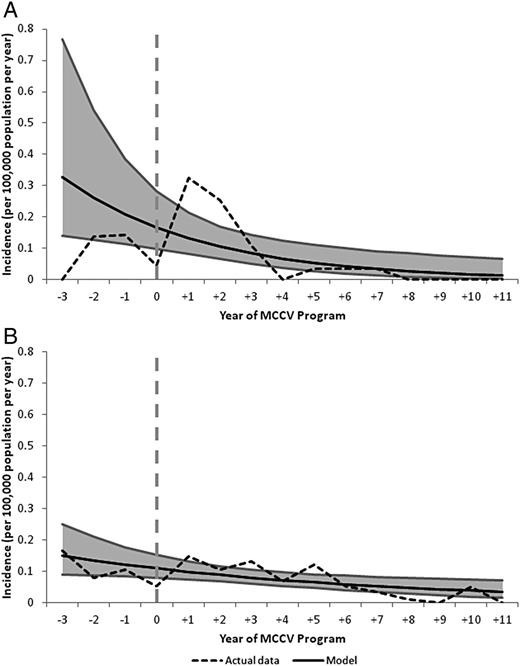
The incidence in those targeted for vaccination was 0.14 per 100 000 per year prior to MCCV introduction, compared to a lower incidence of 0.10 per 100 000 per year in populations who were not included in the immunization schedule. A reduction in serogroup C disease was seen in both vaccinated and unvaccinated populations. In the vaccinated, directly protected population there was an 87% reduction in incidence during the study period (P = .0049) and a decrease of 46% in the unvaccinated, indirectly protected population (P = .0107; Figure 4).
A and B, Actual incidence rates and Poisson regression models of incidence of serogroup C IMD from year −3 to +11 of MCCV Program in all provinces in individuals who were in age groups targeted for vaccination and benefited from direct protection from the vaccine (A) and in individuals who were not in age groups targeted for vaccination and benefited from indirect protection from the vaccine (B). Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
Cases of Serogroup C Disease in Recipients of MCCV
There were a total of 9 cases of serogroup C IMD in individuals who had previously been vaccinated with MCCV (Table 2). One child received a dose of vaccine 4 days prior to admission so would not be expected to be protected. Three children who were approximately 3 years of age when they had IMD had received 2 doses of vaccine in early infancy but missed the 12-month dose. Two children (aged 2 years and 3 years) had received a single dose of MCCV at 12 months. Three children had received a single dose of vaccine between 3 years and 14 years of age and developed disease just over 5 years post-vaccination.
Cases of Serogroup C Invasive Meningococcal Disease (IMD) in Individuals Who Had Previously Received Meningococcal Serogroup C Conjugate Vaccine (MCCV)
| Case Number . | Year of MCCV Program . | Number of MCCV Doses Received . | Age When Doses Received . | Age of Child When Admitted With IMD . | Interval Between Last Dose of MCCV and IMD . |
|---|---|---|---|---|---|
| 1 | +2 | 1 | 12 mos | 12 mo | 4 d |
| 2 | +3 | 2 | 2, 4 mo | 3 y 2 mo | 2 y 10 mo |
| 3 | +3 | 2 | 4, 6 mo | 2 y 9 mo | 2 y 3 mo |
| 4 | +3 | 2 | 5, 6 mo | 2 y 11 mo | 2 y 5 mo |
| 5 | +4 | 1 | 12 mo | 2 y | 12 mo |
| 6 | +6 | 1 | 12 mo | 3 y 3 mo | 2 y 3 mo |
| 7 | +7 | 1 | 3 y 6 mo | 8 y 11 mo | 5 y 5 mo |
| 8 | +2 | 1 | 6 y 10 mo | 11 y 11 mo | 5 y 1 mo |
| 9 | +5 | 1 | 14 y 4 mo | 19 y 9 mo | 5 y 5 mo |
| Case Number . | Year of MCCV Program . | Number of MCCV Doses Received . | Age When Doses Received . | Age of Child When Admitted With IMD . | Interval Between Last Dose of MCCV and IMD . |
|---|---|---|---|---|---|
| 1 | +2 | 1 | 12 mos | 12 mo | 4 d |
| 2 | +3 | 2 | 2, 4 mo | 3 y 2 mo | 2 y 10 mo |
| 3 | +3 | 2 | 4, 6 mo | 2 y 9 mo | 2 y 3 mo |
| 4 | +3 | 2 | 5, 6 mo | 2 y 11 mo | 2 y 5 mo |
| 5 | +4 | 1 | 12 mo | 2 y | 12 mo |
| 6 | +6 | 1 | 12 mo | 3 y 3 mo | 2 y 3 mo |
| 7 | +7 | 1 | 3 y 6 mo | 8 y 11 mo | 5 y 5 mo |
| 8 | +2 | 1 | 6 y 10 mo | 11 y 11 mo | 5 y 1 mo |
| 9 | +5 | 1 | 14 y 4 mo | 19 y 9 mo | 5 y 5 mo |
Case 1 cannot be considered a true vaccine failure due to the short interval between vaccination and disease. Cases 2–4 occurred in children who only received 2 doses of MCCV in infancy and missed the 12-month booster, developing IMD 2–3 years after their last dose of vaccine. Cases 5 and 6 only received a single 12-month dose of vaccine and developed disease 1–2 years subsequent to that. Cases 7–9 received a single dose as older children and developed IMD 5–6 years later. Most cases occurred in the first 3–4 years after introduction of MCCV program into a province.
Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
Cases of Serogroup C Invasive Meningococcal Disease (IMD) in Individuals Who Had Previously Received Meningococcal Serogroup C Conjugate Vaccine (MCCV)
| Case Number . | Year of MCCV Program . | Number of MCCV Doses Received . | Age When Doses Received . | Age of Child When Admitted With IMD . | Interval Between Last Dose of MCCV and IMD . |
|---|---|---|---|---|---|
| 1 | +2 | 1 | 12 mos | 12 mo | 4 d |
| 2 | +3 | 2 | 2, 4 mo | 3 y 2 mo | 2 y 10 mo |
| 3 | +3 | 2 | 4, 6 mo | 2 y 9 mo | 2 y 3 mo |
| 4 | +3 | 2 | 5, 6 mo | 2 y 11 mo | 2 y 5 mo |
| 5 | +4 | 1 | 12 mo | 2 y | 12 mo |
| 6 | +6 | 1 | 12 mo | 3 y 3 mo | 2 y 3 mo |
| 7 | +7 | 1 | 3 y 6 mo | 8 y 11 mo | 5 y 5 mo |
| 8 | +2 | 1 | 6 y 10 mo | 11 y 11 mo | 5 y 1 mo |
| 9 | +5 | 1 | 14 y 4 mo | 19 y 9 mo | 5 y 5 mo |
| Case Number . | Year of MCCV Program . | Number of MCCV Doses Received . | Age When Doses Received . | Age of Child When Admitted With IMD . | Interval Between Last Dose of MCCV and IMD . |
|---|---|---|---|---|---|
| 1 | +2 | 1 | 12 mos | 12 mo | 4 d |
| 2 | +3 | 2 | 2, 4 mo | 3 y 2 mo | 2 y 10 mo |
| 3 | +3 | 2 | 4, 6 mo | 2 y 9 mo | 2 y 3 mo |
| 4 | +3 | 2 | 5, 6 mo | 2 y 11 mo | 2 y 5 mo |
| 5 | +4 | 1 | 12 mo | 2 y | 12 mo |
| 6 | +6 | 1 | 12 mo | 3 y 3 mo | 2 y 3 mo |
| 7 | +7 | 1 | 3 y 6 mo | 8 y 11 mo | 5 y 5 mo |
| 8 | +2 | 1 | 6 y 10 mo | 11 y 11 mo | 5 y 1 mo |
| 9 | +5 | 1 | 14 y 4 mo | 19 y 9 mo | 5 y 5 mo |
Case 1 cannot be considered a true vaccine failure due to the short interval between vaccination and disease. Cases 2–4 occurred in children who only received 2 doses of MCCV in infancy and missed the 12-month booster, developing IMD 2–3 years after their last dose of vaccine. Cases 5 and 6 only received a single 12-month dose of vaccine and developed disease 1–2 years subsequent to that. Cases 7–9 received a single dose as older children and developed IMD 5–6 years later. Most cases occurred in the first 3–4 years after introduction of MCCV program into a province.
Abbreviations: IMD, invasive meningococcal disease; MCCV, meningococcal serogroup C conjugate vaccine.
DISCUSSION
This study has demonstrated that MCCV has resulted in a dramatic and sustained reduction in serogroup C meningococcal disease across Canada. If this effect is representative of the entire country, 75–85 IMD cases and 10–12 deaths will have been prevented in Canada annually [10]. There has been no evidence of serogroup replacement overall, and a reduction in disease in all provinces irrespective of the schedule used, suggesting that the doses at 12 months and in adolescence may be the most important (and perhaps sufficient) since these were almost universally included in immunization schedules. This study has provided clear evidence that this vaccine likely induced herd immunity by reducing the transmission of N. meningitidis, protecting those in the population who were not vaccinated and likely provided longer lasting population protection by prevention of meningococcal carriage.
The reduction following introduction of MCCV in Canada is similar to other countries. In Europe there was a 10-fold drop in the incidence of serogroup C IMD in the 4–7 years after vaccine introduction in 6 countries [11]. These countries used 2–3 doses in infants or a single dose at 12 months, with a catch-up campaign and no routine adolescent dose. In the United Kingdom there was a decrease of 99% in serogroup C disease from 1.85 to 0.02 per 100 000 per year in the 10 years following vaccine introduction, initially using a schedule of 3 doses in early infancy and a mass immunization campaign of everyone up to 24 years of age [12]. This was subsequently changed to 2 infant doses plus a 12-month dose, similar to the initial schedule used in Alberta. The decrease in the United Kingdom is similar to Canada in provinces where such longer-term data are available. In the Netherlands, children receive a single dose of MCCV at 14 months of age, although a catch-up campaign of all children up to 18 years was undertaken when MCCV was introduced—similar to the program in Quebec. In 8 years following MCCV introduction, there was a decrease in serogroup C incidence from 1.7 to 0.03–0.07 per 100 000 per year [13]. Similar successes have also been achieved in Spain and Australia, but neither of these countries use an adolescent dose [14].
MCCV has been a highly successful vaccine globally, in large part due to its ability to induce herd immunity by reducing nasopharyngeal carriage of N. meningitidis and consequent interruption of transmission, primarily in adolescents and young adults [15]. Inclusion of an adolescent dose of vaccine to include this age group has therefore been a critical strategic decision during vaccine implementation. Although the presence of a routine adolescent dose should maintain population herd immunity, the effect of a 1-off catch-up campaign has the potential to diminish over time due to waning of immunity in those immunized as young children [13]. The cases of disease in vaccinated individuals in this study confirm what others have demonstrated regarding persistence of immunity following vaccination with MCCV. Overall, 8/157 cases (5.1%) occurred in vaccinated children (excluding the child who developed disease 4 days post-vaccine). This is similar to data from the United Kingdom, where there were 53 cases in vaccinated children [16] in the first 3 years after introduction of MCCV of a total of 1294 serogroup C cases (4.1%) [17]. In the majority of children, protection following doses in early infancy does not persist beyond 12 months of age [18], whereas immunity is maintained for 1–2 years after a dose at 12 months of age [13], 2–5 years for those vaccinated between 1 year and 9 years of age, and at least 5 years if vaccinated when 10 years or older [19–22].
All provinces and territories in Canada have had a routine adolescent meningococcal vaccine dose in place since 2007, with the exception of Alberta (introduced in 2010) and Quebec (2013). This should ensure the current low incidence of serogroup C disease in Canada persists, as long as the high coverage required to sustain herd immunity can be achieved. This appears particularly relevant to serogroup C because the highest incidence rate was observed in the 15–24 year age group. In Europe only Austria, Switzerland, and the United Kingdom currently have a routine adolescent dose [23, 24]. The importance of the adolescent booster is also suggested in this study with 4 cases in Alberta in 2012 occurring in unvaccinated individuals in this age group, where the adolescent dose was introduced in 2010, 8 years after MCCV was introduced. It will be important to observe whether serogroup C IMD reemerges in those countries where incidence was previously high and an adolescent dose is not used.
The major strengths of this study are that it was based on active, population-based surveillance to maximize case ascertainment and provides accurate estimates of disease incidence. Inclusion of several provinces and various MCCV schedules has enabled comparison of the different strategies, which can be used to inform future policy decisions in Canada and internationally. The study also has some limitations. Some individuals with IMD residing within the defined study population area may have attended hospitals outside the IMPACT network, leading to an underestimate of disease incidence. If individuals were too sick to have appropriate samples taken early in their illness or they died before samples could be obtained, the bacterium would not be isolated, and such cases would be excluded. The study population areas are generally in urban areas, so data from cases in rural areas were limited. Although it is possible that epidemiology may be different in rural Canada, trends are similar to data collected by the National Enhanced Invasive Meningococcal Disease Surveillance System [10], suggesting that these results are a true reflection of Canadian epidemiology. Systematically collected vaccine uptake data were not available to include in the analyses to provide further support to the conclusion that reduction in serogroup C disease was due to implementation of MCCV. Available data from some provinces show that uptake of MCCV has been high at 82%–96% [9, 25–29], suggesting that MCCV has led to the decreased disease incidence. The number of cases of serogroup C IMD is relatively small, particularly in the 6 month to 2 year age group. Therefore, analyses in some subgroups, including those aged <15 years, did not achieve statistical significance although a downward trend was always observed. However, statistical significance was still achieved for most of the analyses, suggesting that effects were large enough for robust conclusions to be drawn.
The reduction of serogroup C IMD has been a great success story for immunization. The variable implementation of MCCV in different provinces has provided a unique opportunity to compare different policies and as data have emerged about this vaccine, so the vaccination strategies have evolved. Following the successful control of serogroup C IMD, future attention will focus on reduction of disease caused by other serogroups, primarily serogroup B, which is now the leading cause of IMD in Canada [30]. A new vaccine based on subcapsular proteins found within the bacterial outer membrane has recently been licensed in Europe [31] and Canada (www.hc-sc.gc.ca). Quadrivalent meningococcal conjugate vaccines for serogroups A, C, Y, and W are currently used in several provinces and territories as the adolescent dose as an alternative to MCCV. Adequate individual surveillance, particularly in childhood, is also needed to maintain the current low rates of serogroup C disease, to ensure children receive all recommended doses.
Notes
Acknowledgments. The authors gratefully acknowledge the expert assistance provided by the Monitor Liaison (Heather Samson), the IMPACT nurse monitors and staff of the data center (Kim Marty, Wenli Zhang, Shu Yu Fan and Debbe Heayn), the National Microbiology Laboratory (Jianwei Zhou), and our public health and infectious disease colleagues. They thank the Directors and staff of the provincial and territorial public health laboratories for providing the isolates for this study.
Financial support. The Canadian Immunization Monitoring Program, Active (IMPACT) is a national surveillance initiative managed by the Canadian Pediatric Society (CPS) and conducted by the IMPACT network of pediatric investigators. From 2002 to 2011 IMPACT meningococcal surveillance was supported by a grant from Sanofi-Pasteur and from 2012 to 2015 by a grant from Novartis Vaccines & Diagnostics Canada to the CPS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Potential conflicts of interest. J. A. B. is supported by a Career Investigator Award from the Michael Smith Foundation for Health Research, and has received speaker honoraria from Novartis Vaccines and Baxter Inc. M. S. is a coinvestigator on investigator-initiated research grants from Pfizer relating to meningitis. S. A. H. has undertaken clinical trials on meningococcal vaccines funded by Novartis and GlaxoSmithKline and has served on ad hoc advisory boards for both companies. All other authors report no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
APPENDIX
IMPACT network investigators from 2002 to 2012 included the following: N. Bridger and R. Morris, Janeway Children's Health and Rehabilitation Centre, St. John's, Canada; S. Halperin and K. Top, IWK Health Center, Halifax, Canada; P. Déry, Centre Mère-Enfant de Québec, Québec City, Canada; D. Moore, Montreal Children's Hospital, Montreal, Canada; M. Lebel, Hôpital Ste-Justine pour les enfants, Montreal, Canada; N. Le Saux, Children's Hospital of Eastern Ontario, Ottawa, Canada; D. Tran and L. Ford-Jones, The Hospital for Sick Children, Toronto, Canada; J. Embree and B. Law, Winnipeg Children's Hospital, Winnipeg, Canada; R. Tsang, National Microbiology Laboratory, Winnipeg, Canada; B. Tan, Royal University Hospital, Saskatoon, Canada; W. Vaudry, Stollery Children's Hospital, Edmonton, Canada; T. Jadavji and O. G. Vanderkooi, Alberta Children's Hospital, Calgary, Canada; D. Scheifele, L. Sauvé, and J. Bettinger, BC Children's Hospital, Vancouver, Canada.
Author notes
IMPACT network investigators members are given in Appendix Section.





Comments