-
PDF
- Split View
-
Views
-
Cite
Cite
Yasir Lal, Aristides P. Assimacopoulos, Two Cases of Daptomycin-Induced Eosinophilic Pneumonia and Chronic Pneumonitis, Clinical Infectious Diseases, Volume 50, Issue 5, 1 March 2010, Pages 737–740, https://doi.org/10.1086/650487
Close - Share Icon Share
Abstract
We present 2 elderly patients who developed lung infiltrates associated with eosinophilia during intravenous daptomycin treatment. Both patients improved quickly after daptomycin was stopped and steroid treatment was initiated. However, complete recovery did not occur, and both patients became chronically steroid dependent.
Daptomycin is a cyclic lipopeptide with excellent coverage for gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci [1]. Although a prospective study of 1092 patients with complicated skin infections did not demonstrate that daptomycin caused pulmonary adverse effects more than penicillinase-resistant penicillins and vancomycin [2], there have been a few case reports of serious pulmonary complications associated with daptomycin published recently [3–7]. The exact mechanism of lung injury and associated eosinophilia is unknown. There is insufficient long-term follow-up information available for most reported cases.
We describe 2 cases of acute respiratory failure and pulmonary infiltrates associated with eosinophilia during daptomycin treatment. Both patients improved dramatically after daptomycin was stopped and steroid therapy was initiated. In contrast to published reports, however, both of these patients developed chronic pulmonary problems and became steroid dependent over a prolonged period of follow-up.
Case 1. An 82-year-old man with a history of chronic lymphocytic leukemia, stage 3 chronic kidney disease, and ulcerative colitis was admitted to our hospital for fever and dyspnea. A few months prior he had undergone elective right ankle replacement surgery. Because of prosthetic joint infection, his ankle hardware had been removed, and an antibiotic spacer had been placed. Some skin grafting was required to achieve wound closure. The patient was empirically treated with vancomycin and ertapenem but was changed to daptomycin after penicillin-resistant Streptococcus mitis grew from a wound culture and he developed a rash attributable to vancomycin.
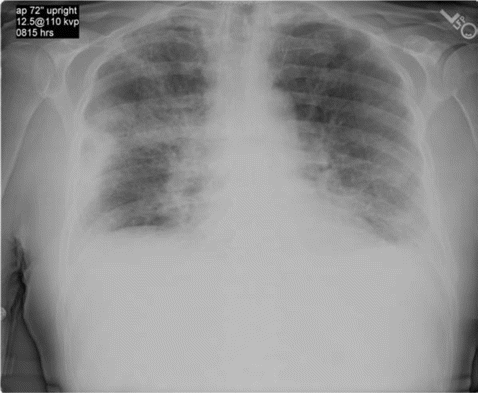
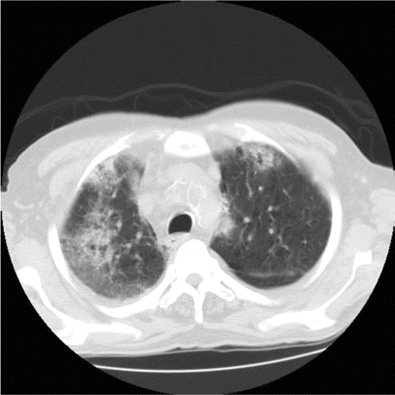
The patient remained asymptomatic until week 3 of daptomycin therapy when he developed fever and was hospitalized. His chronic lymphocytic leukemia and ulcerative colitis were thought to be in remission, and he was not receiving any active treatment at presentation. There was no history of asthma or smoking. He had a temperature of 38°C. His physical exam revealed bibasilar crackles, and his ankle wound was uninfected. Hemogram revealed white blood cell count of 14.9×103 cells/µL (normal range, 4.5×103 to 11.0×103 cells/µL) with significant eosinophilia (1.64×103 eosinophils/µL; normal range, 0.00×103 to 0.45×103 eosinophils/µL). Chest radiography and computed tomography of the chest revealed patchy bilateral infiltrates (Figure 1 and 2). He became more hypoxic over the next 12 h, requiring up to 15 L of oxygen by face mask. Bimodal intermittent airway pressure was used to stabilize his oxygenation. He underwent bronchoscopy with bronchoalveolar lavage. Microscopic examination of bronchoalveolar lavage fluid revealed large numbers of inflammatory cells with 14% eosinophils, and all bacterial viral and fungal cultures were negative.
Chest radiography reveals bilateral patchy infiltrates mostly in peripheral lung fields.
Daptomycin was stopped and high-dose corticosteroids were administered. After 5 days of treatment, hypoxia resolved and the patient was asymptomatic. He was discharged on a short steroid taper. However, soon after completion of his steroid taper, he became progressively more short of breath, and medical evaluation revealed worsened hypoxia and bilateral pulmonary infiltrates. High-dose steroids were re-started, and after recurrent exacerbations, the patient is being maintained on low-dose steroids.
Case 2. The second patient was an 87-year-old man with a history of deep venous thrombosis, pulmonary embolus, and stage 3 chronic kidney disease who developed right prosthetic knee joint infections. He had undergone multiple failed revisions and had previously been received chronic suppressive antibiotic therapy with trimethoprim-sulfamethoxazole for the last several months. After a recent arthroscopy and lavage of the right knee he had been receiving daptomycin therapy for ∼4 weeks at presentation.
He was admitted to a local hospital for 2 weeks because of shortness of breath, dry cough, malaise, anorexia, chills, fever, and leukocytosis. There was no previous history of smoking or asthma. Chest radiography and spiral chest computed tomography demonstrated bilateral patchy pulmonary infiltrates without evidence of pulmonary embolism.
He was empirically treated with levofloxacin but did not improve and was subsequently switched to ceftriaxone and azithromycin. Despite these measures, he did not improve and continued to have fevers with a temperature of 37.8°C–38.4°C, mild leukocytosis, and pleuritic chest pain. He gradually became more dyspneic and was transferred to our facility.
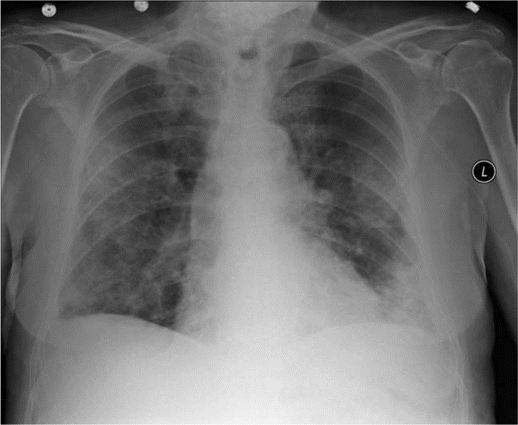
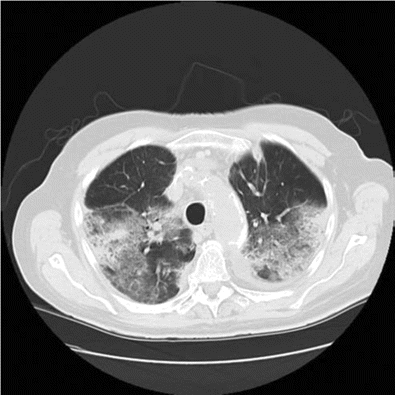
On arrival, he was hypoxic, requiring 15 L of oxygen by face mask to maintain oxygen saturations of ⩾90%. His hemogram revealed a white blood cell count of 14.2×103 cells/µL (normal range, 4.5×103 to 11.0×103 cells/µL) with an eosinophil count of 1.3×103 eosinophils/µL (normal range, 0.00×103 to 0.45×103 eosinophils/µL). Chest radiography and computed tomography were repeated and confirmed the presence of bilateral patchy infiltrates (Figure 3 and 4). Bronchoalveolar lavage was performed. The bronchoalveolar lavage fluid revealed a large number of nucleated cells with 40% eosinophils. Bacterial, viral, and fungal cultures were negative.
Chest radiography reveals bilateral patchy infiltrates mostly in peripheral lung fields.
Computed tomography of the chest reveals ground glass appearance.
Daptomycin was stopped, and parenteral steroids were started. Dramatic recovery occurred within the next 24 h. He was discharged with a tapering dosage of oral prednisone. Shortly after the steroid taper was completed, he developed recurrent dyspnea and pulmonary infiltrates. These resolved partially after further treatment with oral prednisone. He continued to require low-dose steroids for the next 2 years of follow-up.
Discussion. Eosinophilic pneumonitis is a reasonably rare entity. Most commonly, its cause remains uncertain, although sometimes some environmental factor or a drug can be identified as its trigger. Pulmonary infiltrates with eosinophilia have been reported as adverse effects from several drugs, with antibiotics and nonsteroidal anti-inflammatory drugs being the most common trigger [9]. Drug-induced eosinophilic pneumonitis can present either as an acute or chronic syndrome that may occur within days to weeks of starting the inciting agent [8]. In its acute presentation, eosinophilic pneumonitis can resemble acute lung injury (PaO 2 /FiO 2 <300 mmHg) or acute respiratory distress syndrome (PaO 2 /FiO 2 <200 mmHg). Some patients may require mechanical ventilation; however, extra pulmonary organ failure is very rare [10]. Peripheral eosinophilia may not always be present early in the course of disease. Because both patients presented several days after the onset of symptoms, peripheral eosinophilia was present and provided a clue to the diagnosis. However, the absence of eosinophilia does not exclude the diagnosis of drug-induced eosinophilic pneumonitis. To make the diagnosis of drug-induced eosinophilic pneumonitis, one should demonstrate evidence of pneumonitis with a significant eosinophilia in bronchoalveolar lavage fluid and a temporal association with a drug having the potential to cause this syndrome. Of course, infectious causes of eosinophilia and pulmonary infiltrates, such as fungal or parasitic infections, should be excluded. Clinical improvement should occur with cessation of the offending agent, and symptoms should recur after a rechallenge [9]. However, rechallenge with the potential inciting agent is not recommended. Drug-induced eosinophilic pneumonias can have various radiologic presentations. However, most commonly it causes areas of consolidation and ground-glass opacity, usually involving the outer one-third of the lung fields [11]. Lung biopsy can confirm the diagnosis but is not always necessary given a classic clinical presentation and consistent laboratory and imaging studies [9].
The mainstay of treatment of eosinophilic pneumonitis is discontinuation of the inciting agent with administration of steroids. Patients may require respiratory support with supplemental oxygen and assisted ventilation. Most patients will respond within hours of initiation of steroid therapy [7].
The eosinophilic pneumonitis described in these 2 cases is most likely attributable to an adverse drug reaction to daptomycin. Neither patient had any prior history of primary pulmonary disease. Both developed hematologic and pulmonary abnormalities after daptomycin was started and had significant improvement after daptomycin was stopped. All previously published cases progressed to complete resolution after the inciting drug was stopped and steroid therapy was initiated. These cases are unique in that both patients remained steroid dependent during a prolonged follow-up period after the initial episode, similar to patients with a chronic pneumonitis pattern.
Daptomycin's mechanism of action is calcium dependent. It is known to bind synthetic surfactant, reducing its efficacy [12]. Phy et al [4] recently suggested that accumulation of daptomycin in alveolar epithelium could explain its pulmonary adverse effects. On the basis of our observations in these 2 cases, we speculate further that this accumulation can cause long-term sequelae in older patients with lower creatinine clearance. Animal studies could be helpful in further understanding these observations. Additionally, it is not uncommon for a rare adverse effect, such as liver necrosis due to trovafloxacin or telithromycin, not to be seen in premarketing clinical studies but then be noticed when a drug is used more heavily. Therefore, further monitoring of daptomycin use for respiratory adverse effects in the community is advised.
In conclusion, daptomycin is a relatively new drug and is being used more frequently in hospital and intensive care practice. Severe respiratory failure can occur because of drug-induced eosinophilic pneumonitis. Therefore, early recognition is important in potential cases of eosinophilic pneumonitis secondary to receipt of daptomycin. Significant morbidity and mortality can occur if this condition goes unrecognized and is not treated in a timely fashion. As we demonstrate here, some patients can develop chronic pneumonitis and require long-term steroids for relief.
Acknowledgments
Potential conflicts of interest. Y.L. and A.P.A.: no conflicts.





Comments